Cholinesterase Inhibitors: Including Insecticides and Chemical Warfare Nerve Agents
Part 4 - Section 11
Management Strategy 3: Medications
Atropine
Course: WB 1098
CE Original Date: October 16, 2007
CE Renewal Date: October 16, 2010
CE Expiration Date: October 16, 2012
Download Printer-Friendly version [PDF - 1.88 MB]
| Previous Section | Next Section |
Learning Objectives |
Upon completion of this section, you should be able to identify the:
|
||||||||||||||||||||||||||||||||
Atropine: Mechanism of Action |
Muscarinic effectsAtropine works by competitively occupying muscarinic receptor sites, thus reducing the effects of excessive acetylcholine on these sites brought about by cholinesterase inhibition. Nicotinic effectsAtropine is not thought to have significant effect on nicotinic receptors, and thus does not counteract fasciculations, weakness, or flaccid paralysis. (Leikin, Thomas et al. 2002) Thus, even when given sufficient doses of atropine, patients may need artificial ventilation, sometimes for weeks. (Singh, Batra et al. 1995) Note: Although some have suggested that atropine does not cross the blood-brain barrier to any significant extent, (Finkelstein, Kushnir et al. 1989) others disagree, having noted prompt resolution of CNS symptoms after its administration. (Sofer, Tal et al. 1989; De Wilde, Vogelaers et al. 1990) |
||||||||||||||||||||||||||||||||
Diagnostic Use: The “Atropine Challenge” |
A number of authors have recommended the “atropine challenge” as an aid to diagnosis. When given to a normal person who has not been exposed to cholinesterase inhibitors, a 2 mg dose of atropine (0.025-0.050/kg in pediatric cases) causes:
Most of these effects will dissipate within 4-6 hours, except blurred near-vision which may persist for 24 hours. (Sidell 1997) It has been suggested that when these physiological changes do not occur with this dose (sometimes referred to as an atropine challenge), this is indicative of cholinesterase inhibitor toxicity. (Clark 2002; Erdman 2004) Cautions
|
||||||||||||||||||||||||||||||||
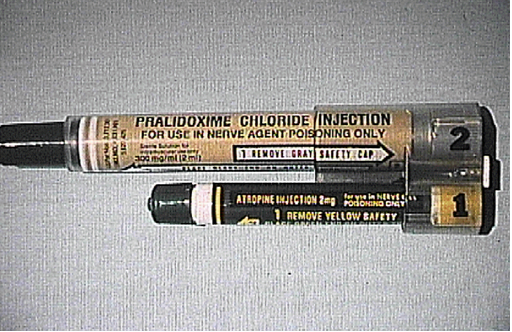
Atropine: Available Forms (Optional Reading) |
The available forms of atropine are
Caution: One author suggest avoiding large doses of pre-mixed atropine containing alcohol preservatives in children out of concern that alcohol toxicity could complicate the situation. (Schenker, Louie et al. 1998) |
||||||||||||||||||||||||||||||||
Atropine: Stability of Solution (Optional Reading) |
There is evidence that atropine solution is very stable and can retain potency long after its expiration date. The table below shows several samples of solution, dating back to World War II, showing its potency. Presumably, the solution was stored in unopened containers (as opposed to multi-dose vials from which any doses had been previously extracted, although this was not explicitly stated. (Schier, Ravikumar et al. 2004)
|
||||||||||||||||||||||||||||||||
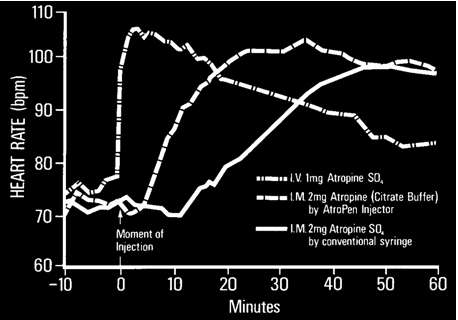
Atropine: Route of Administration |
In approximate order of preference, the following routes of administration can be used for the administration of atropine
|
||||||||||||||||||||||||||||||||
Misconception |
Atropine should be administered until signs of atropinism appear (tachycardia, pupil dilation, and dry mouth). |
||||||||||||||||||||||||||||||||
Reality |
Although atropine has been used to control other, non-life-threatening effects (e.g., nausea and vomiting) the most crucial end-point for atropine dosage titration is control of clinically significant bronchorrhea, bronchoconstriction, (as reflected by level of oxygenation and ease of ventilation) and dangerous bradyarrhythmias or AV-blocks. (Sidell 1997; Reigart and Roberts 1999; Taylor 2001; Erdman 2004; Wiener and Hoffman 2004) |
||||||||||||||||||||||||||||||||
Reasons Why Signs of Atropinism Are Not the Appropriate End Point to Guide Atropine Treatment |
Tachycardia should not be used as an end-point, because it sometimes is a nicotinic manifestation of toxicity.
|
||||||||||||||||||||||||||||||||
Atropine Dose |
AdultsThe most commonly recommended initial doses range from 2 to 6 mg (0.02-0.04 mg/kg). (du Toit, Muller et al. 1981) Authors differ with regards to how frequently these doses should be titrated. Recommended dose intervals vary widely from every 2 to every 30 minutes (Willems 1981; Goswamy, Chaudhuri et al. 1994; Singh, Batra et al. 1995; Carlton, Simpson et al. 1998; Schenker, Louie et al. 1998; Tareg et al. 2001; Erdman 2004; Fernández 2004) (or 1-3 2 mg autoinjector doses). (Sidell 1997) After adequate control of secretions, Du Toit et al. started their patients with an I.V. maintenance drip of 0.02-0.08 mg/kg/hr titrated to effect. (du Toit, Muller et al. 1981) One reported case required 0.5-2.4 mg/kg/hr, I.V. drip, during 5-weeks of treatment. (LeBlanc, Bensen et al. 1986) Some attempts have been made (mostly for nerve agents) to characterize the doses needed according to severity of symptoms. (See the table below for an example.) The goal of therapy with atropine is to reverse life-threatening signs and symptoms (i.e., respiratory distress), and make the patient more comfortable. Currently it is thought that this does not necessarily require the reversal of all effects of the cholinesterase inhibitor [e.g., miosis (pupillary constriction)]. Generally, in patients with severe symptoms, it is better to give too much atropine than too little. (Sidell 1997) Children under 12 years of ageMost authors' recommended doses range from 0.05-0.1 mg/kg boluses q 2-30 min. (Zwiener and Ginsburg 1988; Carlton, Simpson et al. 1998; Reigart and Roberts 1999; Fernández 2004) Pediatric atropine autoinjectors (0.5 mg, 1 mg sizes) (Food and Drug Administration 2003) See the chart below. Intravenous dripRecommendations for I.V. maintenance doses have ranged from 0.2-2.0 mg/hour (0.025 mg/kg/hr in children). (du Toit, Muller et al. 1981; Erdman 2004) OphthalmicTopical mydriatics, such as atropine, and homatropine, can provide relief from eye pain and reflex nausea and vomiting. However, these drugs cause blurring of vision, and should be reserved for cases with severe eye pain. (Sidell 1997) Warning: Hydrocarbons may be used as diluents in liquid formulations of cholinesterase inhibitors. In cases of ingestion, aspiration pneumonitis with acute respiratory distress syndrome may add to the muscarinic respiratory effects of the poison, but is unresponsive to atropine. (Reigart and Roberts 1999) |
||||||||||||||||||||||||||||||||
Misconception |
Atropine dosage requirements for chemical warfare agent toxicity are greater than for cholinesterase-inhibiting pesticides. |
||||||||||||||||||||||||||||||||
Reality |
The dosage requirements for organophosphorus pesticide toxicity, especially with suicidal ingestions, can be higher by orders of magnitude than is the case for nerve agents. |
||||||||||||||||||||||||||||||||
Total Doses of Atropine in Poisoning with Organophosphorus Compounds (Optional Reading) |
Organophosphorus compoundsThe range of atropine doses in the first 12-24 hours stratified by severity is illustrated in the following tables.
|
||||||||||||||||||||||||||||||||
Extremely High Total Doses of Atropine May be Needed in Suicidal Ingestions of Organophosphorus Compounds (Optional Reading) |
In cases of suicidal ingestions, atropine doses in the hundreds of milligrams per day have sometimes been needed. (Wyckoff, Davies et al. 1968; Hopmann and Wanke 1975; du Toit, Muller et al. 1981; Golsousidis and Kokkas 1985; Goswamy, Chaudhuri et al. 1994; Singh, Batra et al. 1995)
|
||||||||||||||||||||||||||||||||
Nerve Agents Require Lower Total Doses of Atropine (Optional Reading) |
In general, severe nerve agent poisoning requires lower total doses of atropine than for organophosphorus compounds. (Sidell 1997) In severe cases (apneic and unconscious), it may require up to 5-15 mg of atropine to restore consciousness and breathing, and atropine has not been required for more than 2-3 hours. (However, distressing, but not life-threatening effects, such as nausea and vomiting, have required atropine for 6-36 hours afterwards). (Sidell 1997) In the Tokyo Sarin attack, only 21 of 107 patients who needed atropine required more than 2 mg, and none required more than 9 mg. (Okumura, Takasu et al. 1996)
|
||||||||||||||||||||||||||||||||
Carbamates Require Lower Doses (Optional Reading) |
While it is difficult to find information on the actual doses of atropine needed, the severity of carbamate poisoning tends to be less than that for organophosphorus compounds. The duration of toxicity also tends to be shorter for most patients; on the order of 6-12 hours. (Carlton, Simpson et al. 1998) Warning: Mixed poisoning with organophosphorus compounds and carbamates are common. In one case series of 52 patients, 35% were mixed exposures. (Carlton, Simpson et al. 1998) |
||||||||||||||||||||||||||||||||
Atropine: Low Incidence of Adverse Effects |
Serious, life-threatening adverse effects from the use of atropine to treat cholinesterase inhibitor toxicity appear to be uncommon. This is even when administered accidentally to children without cholinesterase inhibitor toxicity. Example: In 268 children accidentally autoinjected with high-dose (up to 17 fold higher than recommended for age) in Israel during the Persian Gulf crises, no fatalities, seizures or life-threatening dysrhythmias were observed. (Amitai, Almog et al. 1992) I.V. atropine has caused ventricular fibrillation in hypoxic animals with nerve agent poisoning. Therefore, it has been recommended that hypoxia be corrected if possible prior to atropine administration. However, atropine should not be withheld due to fears of this complication. (Leikin et al. 2002) Excessive doses of atropine can cause a number of mostly minor anticholinergic symptoms (an exception, perhaps, being delirium), though they can last 24-48 hours. Examples include
Note: Physostigmine should NOT be administered for these effects in patients with cholinesterase inhibitor poisoning. (Leikin, Thomas et al. 2002) |
||||||||||||||||||||||||||||||||
Alternatives to Atropine (Optional Reading) |
While other antimuscarinic agents (e.g., scopolamine) can counteract the effects of cholinesterase inhibitors, their inherent toxic effects in patients who do not have cholinesterase inhibitor poisoning have led to their rejection in favor of atropine. (Sidell 1997; Wiener and Hoffman 2004) Glycopyrrolate in doses of 1-2 mg, I.V., (0.025 mg/kg in children) has been suggested as an alternative to atropine, and is said to have fewer CNS side effects. (Clark 2002) However, its use has not been extensively evaluated. (Erdman 2004) |
||||||||||||||||||||||||||||||||
Key Points |
|
||||||||||||||||||||||||||||||||
Progress Check |
|||||||||||||||||||||||||||||||||
| Previous Section | Next Section |