Polycyclic Aromatic Hydrocarbons (PAHs)
What Are Polycyclic Aromatic Hydrocarbons (PAHs)?
Course: WB 1519
CE Original Date: July 1, 2009
CE Renewal Date: July 1, 2011
CE Expiration Date: July 1, 2013
Download Printer-Friendly version [PDF - 546 KB]
Learning Objectives |
Upon completion of this section, you will be able to
- explain what PAHs are, and
- describe the properties of PAHs.
|
Definition |
PAHs are a class of organic compounds produced by incomplete combustion or high-pressure processes. PAHs form when complex organic substances are exposed to high temperatures or pressures.
Often, PAHs consist of three or more fused benzene rings containing only carbon and hydrogen (Figure 1). Differences in the configuration of rings may lead to differences in properties.
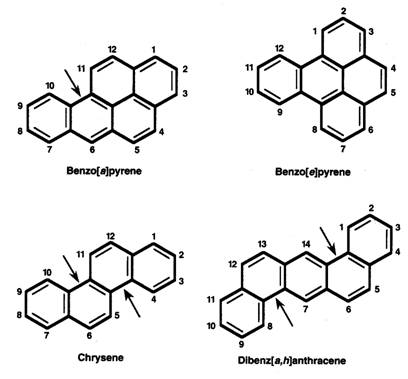
Figure 1. Structural Formulas of Selected Polycyclic Aromatic Hydrocarbons (PAHs). The arrows indicate bay regions.
|
Synonyms |
PAHs are known by several names:
- polycyclic organic matter (POM),
- polynuclear aromatic hydrocarbons,
- polynuclear aromatics (PNAs), and
- polynuclear hydrocarbons.
The more common PAHs include
- benzo(a)anthracene,
- benzo(a)pyrene,
- benzo(e)pyrene,
- benzo(g,h,i)perylene,
- benzo(k)fluoranthene,
- chrysene,
- coronene,
- dibenz(a,h)acridine,
- dibenz(a,h)anthracene, and
- pyrene.
|
Properties |
PAHs:
- are solids with low volatility at room temperature,
- have relatively high molecular weights,
- are soluble in many organic solvents,
- are relatively insoluble in water, and
- most can be photo-oxidized and degraded to simpler substances.
|
Key Points |
- PAHs are a class of organic compounds produced by incomplete combustion or high-pressure processes.
- Often, PAHs consist of three or more fused benzene rings containing only carbon and hydrogen.
- PAHs are solids with low volatility at room temperature. They are relatively insoluble in water, and most can be photo-oxidized and degraded to simpler substances.
|
| |
|
Progress Check |
|



