
Ascariasis
[Ascaris lumbricoides]
Causal Agents
Ascaris lumbricoides is the largest nematode (roundworm) parasitizing the human intestine. (Adult females: 20 to 35 cm; adult male: 15 to 30 cm.)
Life Cycle:

Adult worms  . live in the lumen of the small intestine. A female may produce approximately 200,000 eggs per day, which are passed with the feces
. live in the lumen of the small intestine. A female may produce approximately 200,000 eggs per day, which are passed with the feces  . Unfertilized eggs may be ingested but are not infective. Fertile eggs embryonate and become infective after 18 days to several weeks
. Unfertilized eggs may be ingested but are not infective. Fertile eggs embryonate and become infective after 18 days to several weeks  , depending on the environmental conditions (optimum: moist, warm, shaded soil). After infective eggs are swallowed
, depending on the environmental conditions (optimum: moist, warm, shaded soil). After infective eggs are swallowed  , the larvae hatch
, the larvae hatch  , invade the intestinal mucosa, and are carried via the portal, then systemic circulation to the lungs
, invade the intestinal mucosa, and are carried via the portal, then systemic circulation to the lungs  . The larvae mature further in the lungs (10 to 14 days), penetrate the alveolar walls, ascend the bronchial tree to the throat, and are swallowed
. The larvae mature further in the lungs (10 to 14 days), penetrate the alveolar walls, ascend the bronchial tree to the throat, and are swallowed  . Upon reaching the small intestine, they develop into adult worms . Between 2 and 3 months are required from ingestion of the infective eggs to oviposition by the adult female. Adult worms can live 1 to 2 years.
. Upon reaching the small intestine, they develop into adult worms . Between 2 and 3 months are required from ingestion of the infective eggs to oviposition by the adult female. Adult worms can live 1 to 2 years.
Geographic Distribution:
The most common human helminthic infection. Worldwide distribution. Highest prevalence in tropical and subtropical regions, and areas with inadequate sanitation. Occurs in rural areas of the southeastern United States.
Clinical Presentation
Although infections may cause stunted growth, adult worms usually cause no acute symptoms. High worm burdens may cause abdominal pain and intestinal obstruction. Migrating adult worms may cause symptomatic occlusion of the biliary tract or oral expulsion. During the lung phase of larval migration, pulmonary symptoms can occur (cough, dyspnea, hemoptysis, eosinophilic pneumonitis - Loeffler's syndrome).
Ascaris lumbricoides unfertilized eggs.
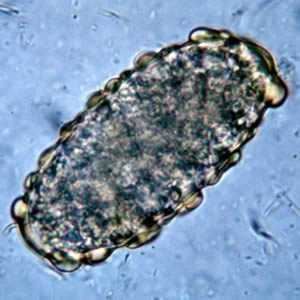
Figure A: Unfertilized egg of A. lumbricoides. Note the prominent mammillations on the outer layer.
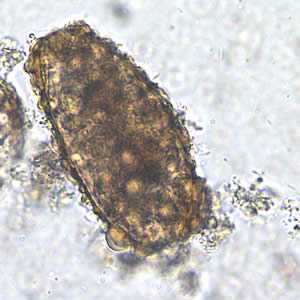
Figure B: Unfertilized egg of A. lumbricoides in an unstained wet mount, 200x magnification.
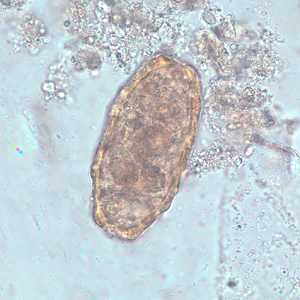
Figure C: Unfertilized egg of A. lumbricoides in an unstained wet mount of stool.

Figure D: Unfertilized egg of A. lumbricoides in a wet mount of stool. Note this specimen lacks the mammillated layer (decorticated).
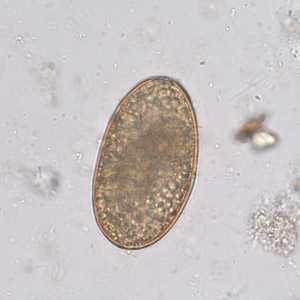
Figure E: Infertile, decorticated egg of Ascaris lumbricoides. Image courtesy of The Leiden University Medical Center, The Netherlands.
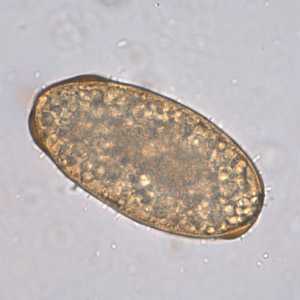
Figure F: Infertile, decorticated egg of Ascaris lumbricoides. Image courtesy of The Leiden University Medical Center, The Netherlands.
A. lumbricoides fertilized eggs.
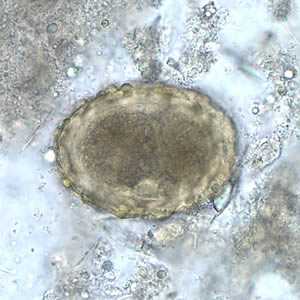
Figure A: Fertilized egg of A. lumbricoides in unstained wet mounts of stool, with embryos in the early stage of development.
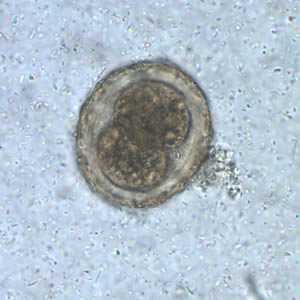
Figure B: Fertilized egg of A. lumbricoides in unstained wet mounts of stool, with embryos in the early stage of development.
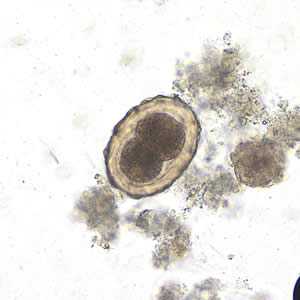
Figure C: Fertilized egg of A. lumbricoides in an unstained wet mount of stool, undergoing early stages of cleavage. Image taken at 200x magnification.
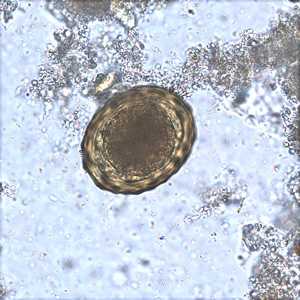
Figure D: Fertilized egg of A. lumbricoides in an unstained wet mount of stool.
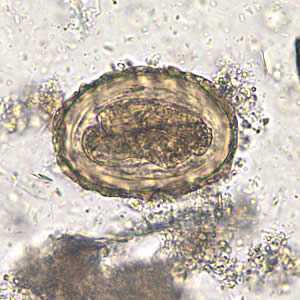
Figure E: Fertilized egg of A. lumbricoides in an unstained wet mount of stool, 200x magnification. A larva is visible in the egg.
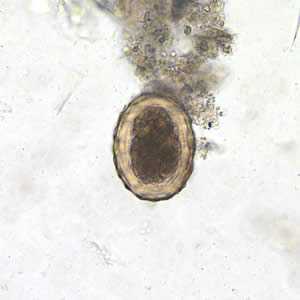
Figure F: Fertilized egg of A. lumbricoides in an unstained wet mount of stool.
A. lumbricoides fertilized, decorticated eggs.

Figure A: A. lumbricoides decorticated, fertile egg in wet mounts, 200x magnification.

Figure B: A. lumbricoides decorticated, fertile egg in wet mounts, 200x magnification.

Figure C: A. lumbricoides decorticated, fertile egg in a wet mount, 200x magnification. The embryo has advanced cleavage.

Figure D: The same egg as in Figure C, but at 400x magnification.
Larvae of A. lumbricoides hatching from eggs.
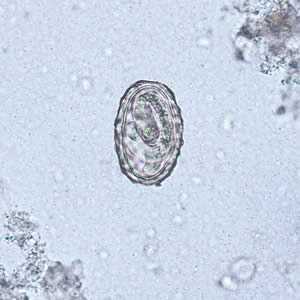
Figure A: Larva of A. lumbricoides hatching from an egg.
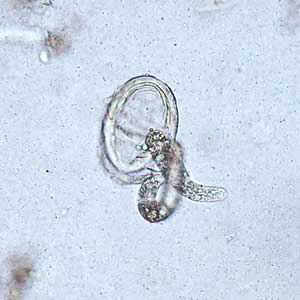
Figure B: Larva of A. lumbricoides hatching from an egg.
Adults of A. lumbricoides.
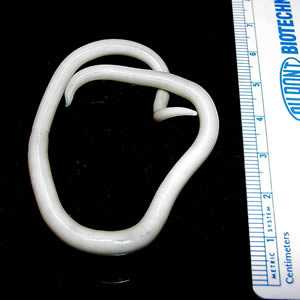
Figure A: Adult female A. lumbricoides.

Figure B: Adult female A. lumbricoides. Image courtesy of the Orange County Public Health Laboratory, Santa Ana, CA.

Figure C: Close-up of the anterior end of an adult A. lumbricoides. Note the three 'lips.' Image courtesy of the Orange County Public Health Laboratory, Santa Ana, CA.
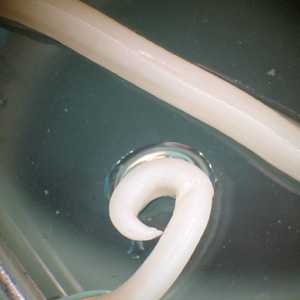
Figure D: Posterior end of a male A. lumbricoides, showing the curled tail.
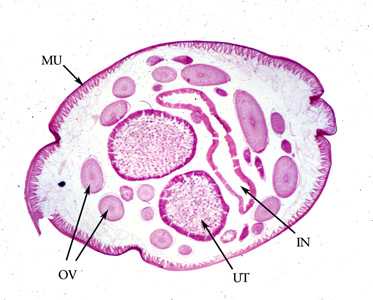
Figure E: Cross-section of an adult female A. lumbricoides, stained with hematoxylin and eosin (H&E). Note the presence of the prominent muscle cells (MU), gravid uterus (UT), intestine (IN) and coiled ovary (OV).
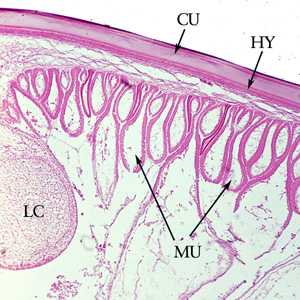
Figure F: Cross-section of the cuticle of an adult A. lumbricoides, stained with H&E. Shown here are the cuticle (CU), and immediately below the cuticle, the thin hypodermis (HY). Also shown are the prominent muscle cells (MU) and one of the lateral chords (LC).
A. lumbricoides in tissue specimens.
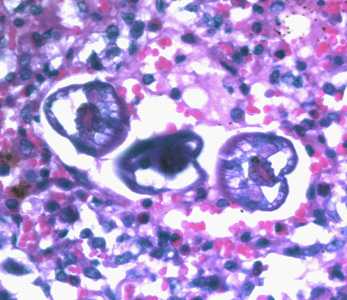
Figure A: L3 larvae of A. lumbricoides in lung tissue, stained with H&E. Image taken at 400x magnification.
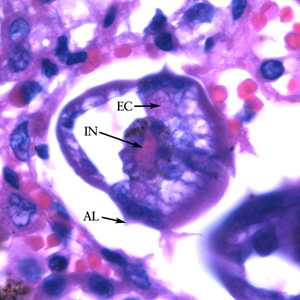
Figure B: Higher magnification (1000x) of the specimen in Figure A. Note the prominent alae (AL), intestine (IN) and excretory ducts (EC).
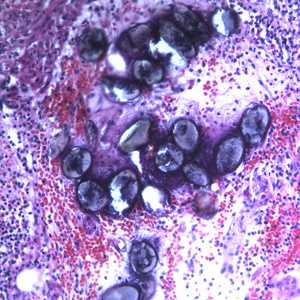
Figure C: Eggs of A. lumbricoides in an appendix biopsy, stained with H&E. This image was taken at 200x magnification.
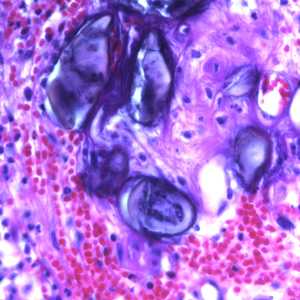
Figure D: Eggs of A. lumbricoides in an appendix biopsy, stained with H&E. This image was taken at 400x magnification.
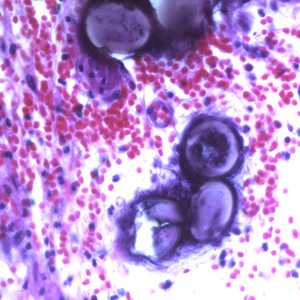
Figure E: Eggs of A. lumbricoides in an appendix biopsy, stained with H&E. Image taken at 400x magnification.
Laboratory Diagnosis
Morphologic Diagnosis
Microscopic identification of eggs in the stool is the most common method for diagnosing intestinal ascariasis. The recommended procedure is as follows:
- Collect a stool specimen.
- Fix the specimen in 10% formalin.
- Concentrate using the formalin–ethyl acetate sedimentation technique
- Examine a wet mount of the sediment.
Where concentration procedures are not available, a direct wet mount examination of the specimen is adequate for detecting moderate to heavy infections. For quantitative assessments of infection, various methods such as the Kato-Katz can be used. Larvae can be identified in sputum or gastric aspirate during the pulmonary migration phase (examine formalin-fixed organisms for morphology). Adult worms are occasionally passed in the stool or through the mouth or nose and are recognizable by their macroscopic characteristics.
Treatment Information
Ascariasis is treated with albendazole, mebendazole, or ivermectin. Dosage is the same for children as for adults. Albendazole should be taken with food. Ivermectin should be taken on an empty stomach with water. Albendazole is not FDA-approved for treating ascariasis, and the safety of ivermectin for treating children who weigh less than 15 kg has not been established.
| Drug | Dosage |
|---|---|
| Albendazole | 400 mg orally once |
| Mebendazole | 100 mg orally twice daily for 3 days or 500 mg orally once |
| Ivermectin | 150-200 mcg/kg orally once |
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
Ivermectin
Oral ivermectin is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: October 17, 2016
- Page last updated: October 17, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir