
Hymenolepiasis
[Hymenolepis diminuta] [Hymenolepis nana]
Causal Agents
Hymenolepiasis is caused by two cestodes (tapeworm) species, Hymenolepis nana (the dwarf tapeworm, adults measuring 15 to 40 mm in length) and Hymenolepis diminuta (rat tapeworm, adults measuring 20 to 60 cm in length). Hymenolepis diminuta is a cestode of rodents infrequently seen in humans and frequently found in rodents.
Life Cycles
Hymenolepis nana
Hymenolepis diminuta
Geographic Distribution
Hymenolepis nana is the most common cause of all cestode infections, and is encountered worldwide. In temperate areas its incidence is higher in children and institutionalized groups. Hymenolepis diminuta, while less frequent, has been reported from various areas of the world.
Clinical Presentation
Hymenolepis nana and H. diminuta infections are most often asymptomatic. Heavy infections with H. nana can cause weakness, headaches, anorexia, abdominal pain, and diarrhea.
Hymenolepis diminuta eggs in wet mounts.
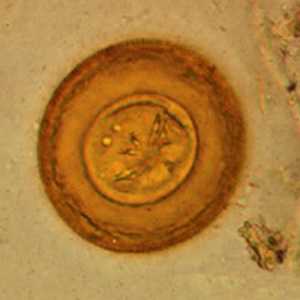
Figure A: Egg of H. diminuta in a wet mount stained with iodine. Four of the hooks are visible at this level of focus. Image courtesy of the Georgia Department of Public Health.
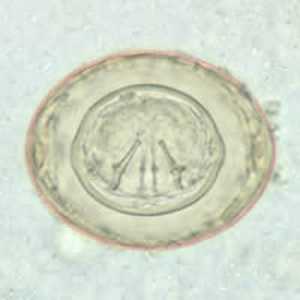
Figure B: Egg of H. diminuta in a wet mount stained with iodine. Four of the hooks are visible at this level of focus.

Figure C: Eggs of H. diminuta in an unstained wet mount of concentrated stool. Image taken at 200x magnification.
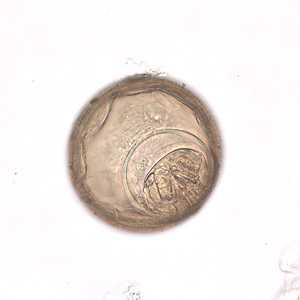
Figure D: Higher magnification (400x) of one of the eggs in Figure C.
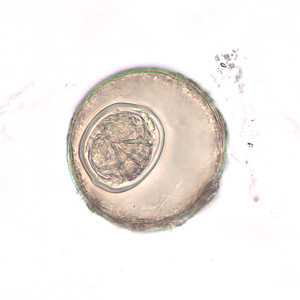
Figure E: Egg of H. diminuta in an unstained wet mount of concentrated stool. Image taken at 400x magnification.

Figure F: Egg of H. diminuta in an unstained wet mount of concentrated stool. Image taken at 400x magnification.
Hymenolepis nana egg in wet mounts.
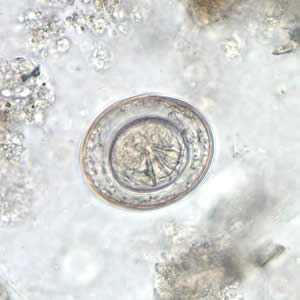
Figure A: Egg of H. nana in an unstained wet mount. Note the presence of hooks in the oncosphere and polar filaments within the space between the oncosphere and outer shell.
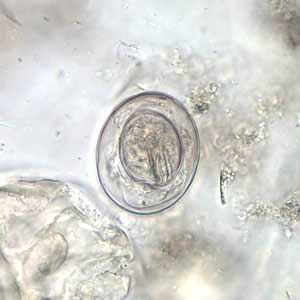
Figure B: Egg of H. nana in an unstained wet mount. Note the presence of hooks in the oncosphere and polar filaments within the space between the oncosphere and outer shell.
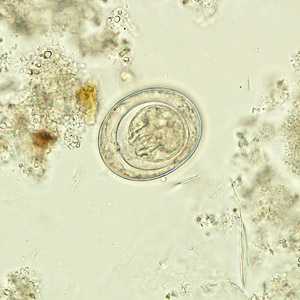
Figure C: Egg of H. nana in an unstained wet mount.
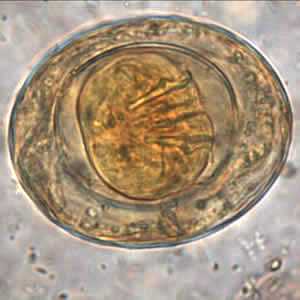
Figure D: Egg of H. nana in an unstained formalin ethyl acetate (FEA) wet mount. In this image, four of the hooks in the oncosphere are clearly visible. Image courtesy of the Oregon State Public Health Laboratory.
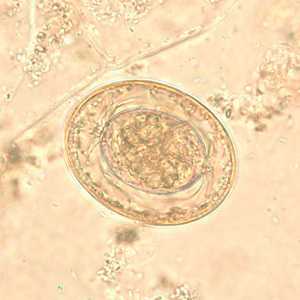
Figure E: Egg of H. nana in an unstained wet mount. In this image, the polar filaments in the space between the oncosphere and outer shell are clearly visible.
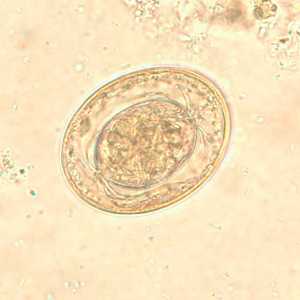
Figure F: Egg of H. nana in an unstained wet mount. In this image, the polar filaments in the space between the oncosphere and outer shell are clearly visible.
Hymenolepis nana eggs, zinc PVA trichrome stain.
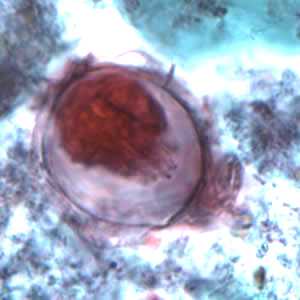
Figure A: Egg of H. nana in a trichrome-stained stool specimen. Although trichrome is not the preferred method for observing helminth eggs, they can be detected this way. The eggs are distorted, probably due to the zinc polyvinyl alcohol (PVA) used for preserving specimens for trichrome stain. Images courtesy of the Oregon State Public Health Laboratory.
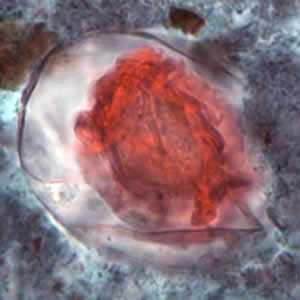
Figure B: Egg of H. nana in a trichrome-stained stool specimen. Although trichrome is not the preferred method for observing helminth eggs, they can be detected this way. The eggs are distorted, probably due to the zinc polyvinyl alcohol (PVA) used for preserving specimens for trichrome stain. Images courtesy of the Oregon State Public Health Laboratory.
Hymenolepis proglottids.
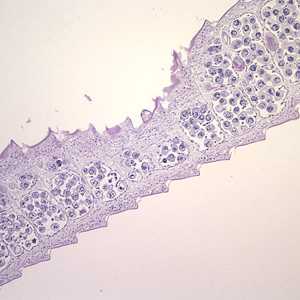
Figure A: Cross-sections of mature proglottids of H. nana stained with hematoxylin and eosin (H&E), taken at 100x. Note the craspedote (overlapping) proglottids.
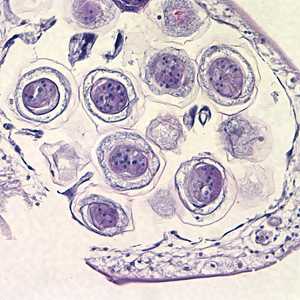
Figure B: Higher magnification of eggs within the proglottid in Figure A, taken at 400x.
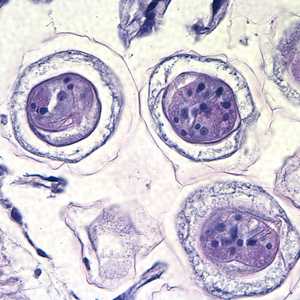
Figure C: Higher magnification of the eggs in Figures A and B, taken at 1000x, oil. Hooks do not stain with H&E but are refractile and may be visible in stained specimens with proper adjustment of the microscope. Polar filaments are visible in the egg in the upper right quadrant of the image.
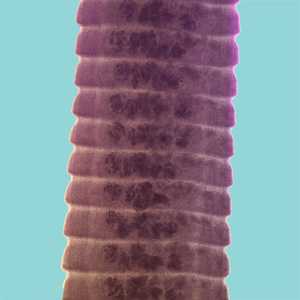
Figure D: Proglottids of H. diminuta stained with carmine. Notice the craspedote form of the proglottids.
Hymenolepis nana adults.
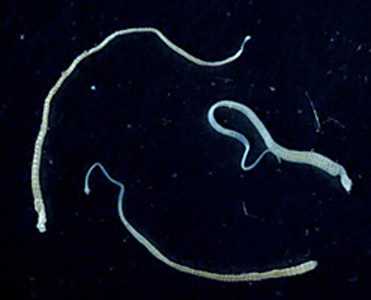
Figure A: Three adult specimens of H. nana. Image courtesy of the Georgia Department of Public Health.
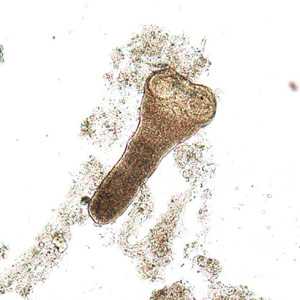
Figure B: Scolex of H. nana in an unstained wet mount of stool. Image courtesy of Dr. David Bruckner.
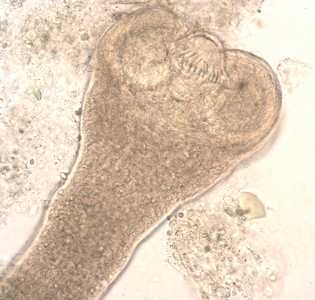
Figure C: Higher magnification of the scolex in Figure B. In this image, two of the suckers and the rostellar hooks are clearly visible.
Intermediate hosts of Hymenolepis spp.

Figure A: Tribolium confusum, a common intermediate host for Hymenolepis spp. Tribolium and related genera breed in cereals, grains, and grain-based snack foods and are easily ingested by humans and rodents. Since these food products are usually not heated prior to consumption, cysticeroids within the beetles remain viable and infective. Image courtesy of Parasite and Diseases Image Library, Australia.

Figure B: Tribolium castaneum, another beetle commonly found in grain products that may serve as an intermediate host for Hymenolepis spp. Image courtesy of Parasite and Diseases Image Library, Australia.
Diagnostic Findings
The diagnosis depends on the demonstration of eggs in stool specimens. Concentration techniques and repeated examinations will increase the likelihood of detecting light infections.
Treatment Information
Praziquantel, adults and children, 25mg/kg in a single-dose therapy.
Alternatives:
Niclosamide*: adults, 2 gm in a single dose for 7 days; children 11-34 kg, 1 gm in a single dose on day 1 then 500 mg per day orally for 6 days; children > 34 kg, 1.5 gm in a single dose on day 1 then 1 gm per day orally for 6 days.
Nitazoxanide: adults, 500 mg orally twice daily for 3 days; children aged 12-47 months, 100 mg orally twice daily for 3 days; children 4-11 years, 200 mg orally twice daily for 3 days.
*Niclosamide is unavailable in the United States.
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
Niclosamide
Niclosamide is NOT available for human use in the United States.
Note on Treatment in Pregnancy
Nitazoxanide
Nitazoxanide is available for human use in the United States.
Note on Treatment in Pregnancy
References
- Chero JC, Saito M, Bustos JA. Hymenolepis infection: symptoms and response to nitazoxanide in field conditions. Trans R Soc Trop Med Hyg 2007;101(2):203-5.
- Craig P, Ito A. Intestinal cestodes. Curr Opin Infect Dis 2007;20:524-32.
- Fox LM, Saravolatz LD. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin Infect Dis 2005;40:1173-80.
- Ortiz JJ, Lopez Chegne N, Gargala G, Favennec L. Comparative clinical studies of nitazoxanide, albendazole and praziquantel in the treatment of ascariasis, trichuriasis and hymenolepiasis in children from Peru. Trans R Soc Trop Med Hyg 2002;96:193-6.
- Jones WE. Niclosamide as a treatment for Hymenolepis diminuta and Dipylidium caninum infection in man. Am J Trop Med Hyg 1979:28(2):300-02.
- Romero Cabello R, Guerrero LR, Munoz Garcia M, Geyne Cruz A. Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Trans R Soc Trop Med Hyg1997:91:701-3.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
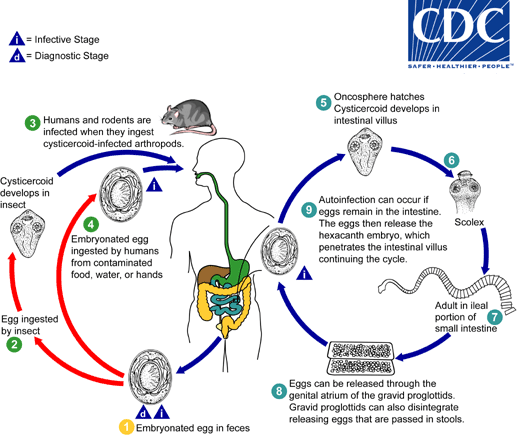
 . When eggs are ingested by an arthropod intermediate host
. When eggs are ingested by an arthropod intermediate host  (various species of beetles and fleas may serve as intermediate hosts), they develop into cysticercoids, which can infect humans or rodents upon ingestion
(various species of beetles and fleas may serve as intermediate hosts), they develop into cysticercoids, which can infect humans or rodents upon ingestion  and develop into adults in the small intestine. A morphologically identical variant, H. nana var. fraterna, infects rodents and uses arthropods as intermediate hosts. When eggs are ingested
and develop into adults in the small intestine. A morphologically identical variant, H. nana var. fraterna, infects rodents and uses arthropods as intermediate hosts. When eggs are ingested  (in contaminated food or water or from hands contaminated with feces), the oncospheres contained in the eggs are released. The oncospheres (hexacanth larvae) penetrate the intestinal villus and develop into cysticercoid larvae
(in contaminated food or water or from hands contaminated with feces), the oncospheres contained in the eggs are released. The oncospheres (hexacanth larvae) penetrate the intestinal villus and develop into cysticercoid larvae  . Upon rupture of the villus, the cysticercoids return to the intestinal lumen, evaginate their scoleces
. Upon rupture of the villus, the cysticercoids return to the intestinal lumen, evaginate their scoleces  , attach to the intestinal mucosa and develop into adults that reside in the ileal portion of the small intestine producing gravid proglottids
, attach to the intestinal mucosa and develop into adults that reside in the ileal portion of the small intestine producing gravid proglottids  . Eggs are passed in the stool when released from proglottids through its genital atrium or when proglottids disintegrate in the small intestine
. Eggs are passed in the stool when released from proglottids through its genital atrium or when proglottids disintegrate in the small intestine  . An alternate mode of infection consists of internal autoinfection, where the eggs release their hexacanth embryo, which penetrates the villus continuing the infective cycle without passage through the external environment
. An alternate mode of infection consists of internal autoinfection, where the eggs release their hexacanth embryo, which penetrates the villus continuing the infective cycle without passage through the external environment  . The life span of adult worms is 4 to 6 weeks, but internal autoinfection allows the infection to persist for years.
. The life span of adult worms is 4 to 6 weeks, but internal autoinfection allows the infection to persist for years.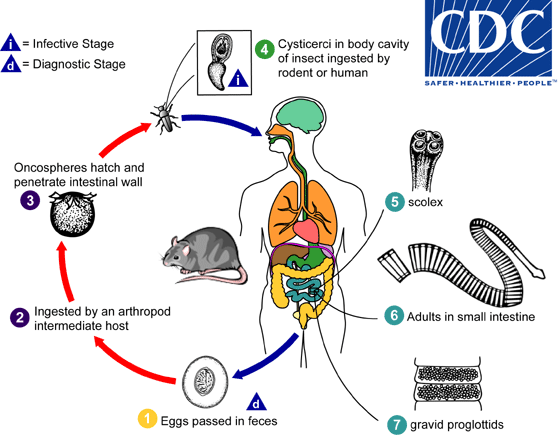
 , and oncospheres are released from the eggs and penetrate the intestinal wall of the host
, and oncospheres are released from the eggs and penetrate the intestinal wall of the host  , which develop into cysticercoid larvae. Species from the genus Tribolium are common intermediate hosts for H. diminuta. The cysticercoid larvae persist through the arthropod's morphogenesis to adulthood. H. diminuta infection is acquired by the mammalian host after ingestion of an intermediate host carrying the cysticercoid larvae
, which develop into cysticercoid larvae. Species from the genus Tribolium are common intermediate hosts for H. diminuta. The cysticercoid larvae persist through the arthropod's morphogenesis to adulthood. H. diminuta infection is acquired by the mammalian host after ingestion of an intermediate host carrying the cysticercoid larvae