
Schistosomiasis Infection
[Schistosoma haematobium] [Schistosoma intercalatum] [Schistosoma japonicum] [Schistosoma mansoni] [Schistosoma mekongi]
Causal Agents
Schistosomiasis is caused by digenetic blood trematodes. The three main species infecting humans are Schistosoma haematobium, S. japonicum, and S. mansoni. Two other species, more localized geographically, are S. mekongi and S. intercalatum. In addition, other species of schistosomes, which parasitize birds and mammals, can cause cercarial dermatitis in humans.
Life Cycle

Eggs are eliminated with feces or urine  . Under optimal conditions the eggs hatch and release miracidia
. Under optimal conditions the eggs hatch and release miracidia  , which swim and penetrate specific snail intermediate hosts
, which swim and penetrate specific snail intermediate hosts  . The stages in the snail include 2 generations of sporocysts
. The stages in the snail include 2 generations of sporocysts  . and the production of cercariae
. and the production of cercariae  . Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host
. Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host  , and shed their forked tail, becoming schistosomulae
, and shed their forked tail, becoming schistosomulae  . The schistosomulae migrate through several tissues and stages to their residence in the veins (
. The schistosomulae migrate through several tissues and stages to their residence in the veins ( ,
,  ). Adult worms in humans reside in the mesenteric venules in various locations, which at times seem to be specific for each species
). Adult worms in humans reside in the mesenteric venules in various locations, which at times seem to be specific for each species  . For instance, S. japonicum is more frequently found in the superior mesenteric veins draining the small intestine
. For instance, S. japonicum is more frequently found in the superior mesenteric veins draining the small intestine  , and S. mansoni occurs more often in the superior mesenteric veins draining the large intestine
, and S. mansoni occurs more often in the superior mesenteric veins draining the large intestine  . However, both species can occupy either location, and they are capable of moving between sites, so it is not possible to state unequivocally that one species only occurs in one location. S. haematobium most often occurs in the venous plexus of bladder
. However, both species can occupy either location, and they are capable of moving between sites, so it is not possible to state unequivocally that one species only occurs in one location. S. haematobium most often occurs in the venous plexus of bladder  , but it can also be found in the rectal venules. The females (size 7 to 20 mm; males slightly smaller) deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S. mansoni and S. japonicum) and of the bladder and ureters (S. haematobium), and are eliminated with feces or urine, respectively
, but it can also be found in the rectal venules. The females (size 7 to 20 mm; males slightly smaller) deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S. mansoni and S. japonicum) and of the bladder and ureters (S. haematobium), and are eliminated with feces or urine, respectively  . Pathology of S. mansoni and S. japonicum schistosomiasis includes: Katayama fever, hepatic perisinusoidal egg granulomas, Symmers’ pipe stem periportal fibrosis, portal hypertension, and occasional embolic egg granulomas in brain or spinal cord. Pathology of S. haematobium schistosomiasis includes: hematuria, scarring, calcification, squamous cell carcinoma, and occasional embolic egg granulomas in brain or spinal cord.
. Pathology of S. mansoni and S. japonicum schistosomiasis includes: Katayama fever, hepatic perisinusoidal egg granulomas, Symmers’ pipe stem periportal fibrosis, portal hypertension, and occasional embolic egg granulomas in brain or spinal cord. Pathology of S. haematobium schistosomiasis includes: hematuria, scarring, calcification, squamous cell carcinoma, and occasional embolic egg granulomas in brain or spinal cord.
Human contact with water is thus necessary for infection by schistosomes. Various animals, such as dogs, cats, rodents, pigs, hourse and goats, serve as reservoirs for S. japonicum, and dogs for S. mekongi.
Geographic Distribution
Schistosoma mansoni is found in parts of South America and the Caribbean, Africa, and the Middle East; S. haematobium in Africa and the Middle East; and S. japonicum in the Far East. Schistosoma mekongi and S. intercalatum are found focally in Southeast Asia and central West Africa, respectively.
Clinical Presentation
Many infections are asymptomatic. Acute schistosomiasis (Katayama's fever) may occur weeks after the initial infection, especially by S. mansoni and S. japonicum. Manifestations include fever, cough, abdominal pain, diarrhea, hepatosplenomegaly, and eosinophilia. Occasionally central nervous system lesions occur: cerebral granulomatous disease may be caused by ectopic S. japonicum eggs in the brain, and granulomatous lesions around ectopic eggs in the spinal cord from S. mansoni and S. haematobium infections may result in a transverse myelitis with flaccid paraplegia. Continuing infection may cause granulomatous reactions and fibrosis in the affected organs, which may result in manifestations that include: colonic polyposis with bloody diarrhea (Schistosoma mansoni mostly); portal hypertension with hematemesis and splenomegaly (S. mansoni, S. japonicum, S. mansoni); cystitis and ureteritis (S. haematobium) with hematuria, which can progress to bladder cancer; pulmonary hypertension (S. mansoni, S. japonicum, more rarely S. haematobium); glomerulonephritis; and central nervous system lesions.
Schistosoma mansoni eggs.
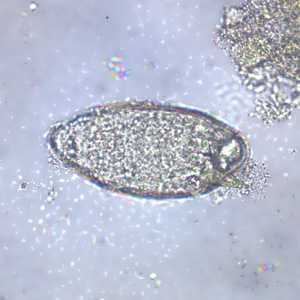
Figure A: Egg of S. mansoni in an unstained wet mount. Images courtesy of the Wisconsin State Laboratory of Hygiene.
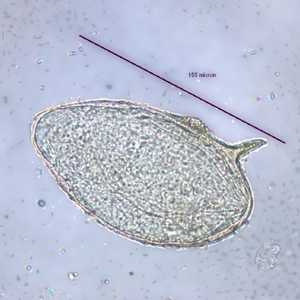
Figure B: Egg of S. mansoni in an unstained wet mount. Images courtesy of the Wisconsin State Laboratory of Hygiene.
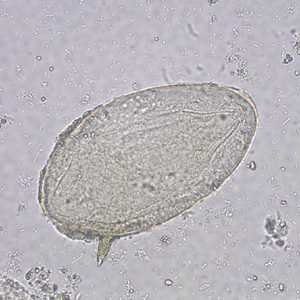
Figure C: Egg of S. mansoni in an unstained wet mount. Images courtesy of the Missouri State Public Health Laboratory.
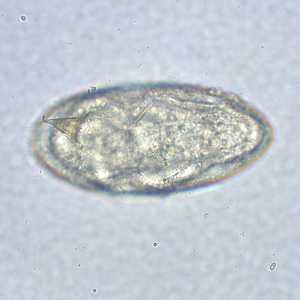
Figure D: Egg of S. mansoni in an unstained wet mount. Images courtesy of the Missouri State Public Health Laboratory.

Figure E: Eggs of S. mansoni in an unstained wet mount.
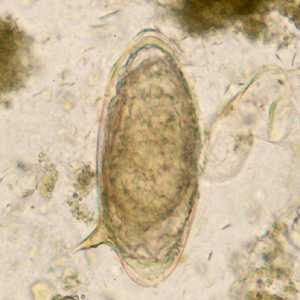
Figure F: Egg of S. mansoni in an unstained wet mount.
Schistosoma haematobium eggs.
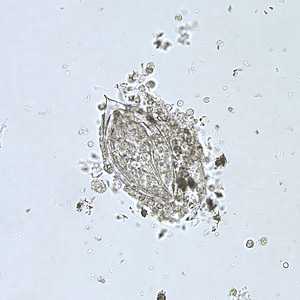
Figure A: Egg of S. haematobium in a wet mount of urine concentrates, showing the characteristic terminal spine.
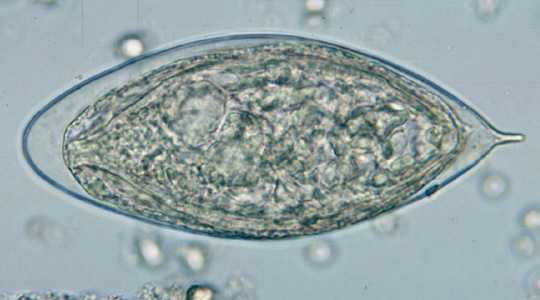
Figure B: Egg of S. haematobium in a wet mount of urine concentrates, showing the characteristic terminal spine.
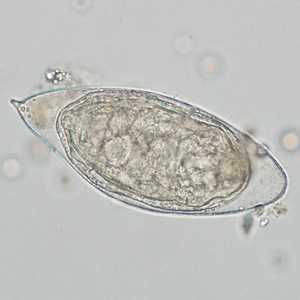
Figure C:Egg of S. haematobium in a wet mount of a urine concentrate.
Schistosoma japonicum eggs.
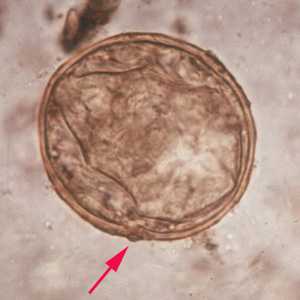
Figure A: Egg of S. japonicum in an unstained wet mount. Note the small, inconspicuous spines (red arrows).
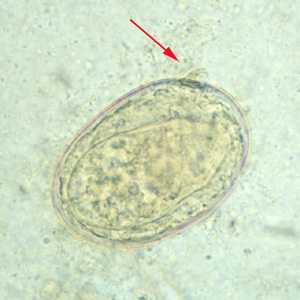
Figure B: Egg of S. japonicum in an unstained wet mount. Note the small, inconspicuous spines (red arrows).
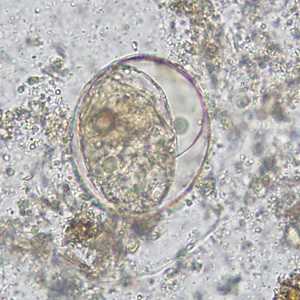
Figure C: Egg of S. japonicum in an unstained wet mount of stool. The spine is not visible in either of these specimens.
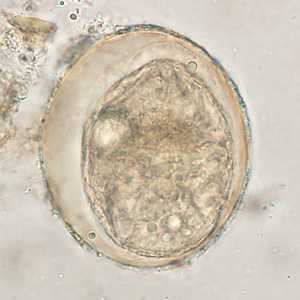
Figure D: Egg of S. japonicum in an unstained wet mount of stool. The spine is not visible in either of these specimens.
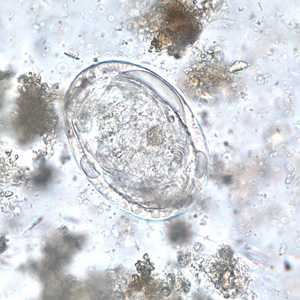
Figure E: Egg of S. Japonicum in an unstained wet mount of stool.
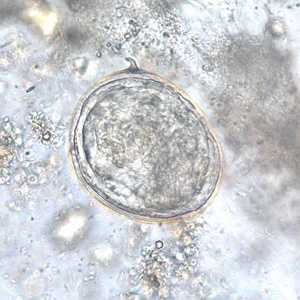
Figure F: Egg of S. japonicum in an unstained wet mount of stool.
Schistosoma intercalatum eggs.
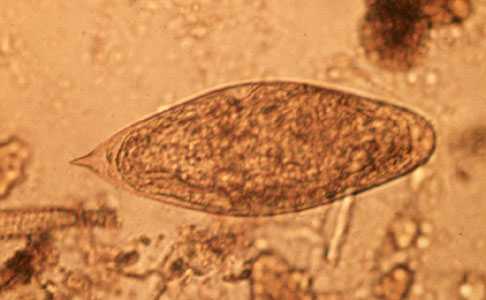
Figure A: Egg of S. intercalatum in a wet mount.
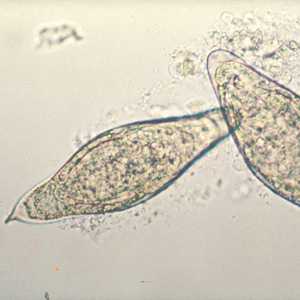
Figure B: Egg of S. intercalatum in a wet mount.
Schistosoma mekongi eggs.
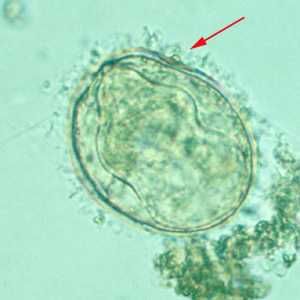
Figure A: Egg of S. mekongi. Note the inconspicuous spine (red arrow).
Schistosoma spp. eggs in tissue, stained with hematoxylin and eosin (H&E).
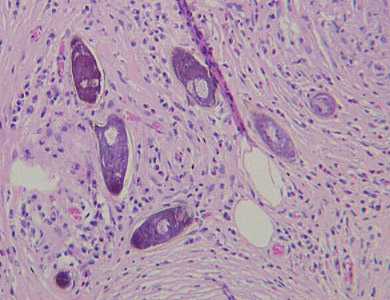
Figure A: Eggs of S. mansoni in liver tissue, stained with H&E. Images courtesy of Dr. Munaf Desai, Al Qassini Hospital, Shatjah, United Arab Emirates.
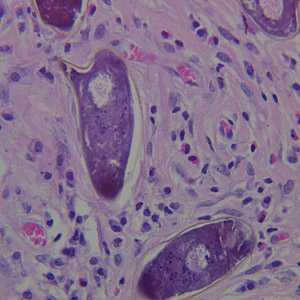
Figure B: Higher magnification of the specimen in Figure A.
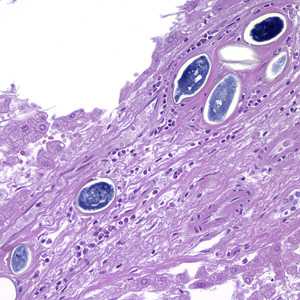
Figure C: Eggs of S. japonicum from tissue, stained with H&E. at 200x magnification.
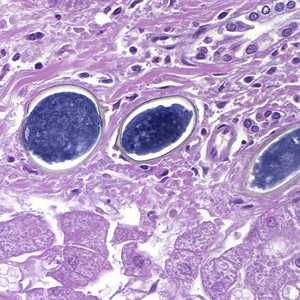
Figure D: Eggs of S. japonicum from tissue, stained with H&E. at 400x magnification.
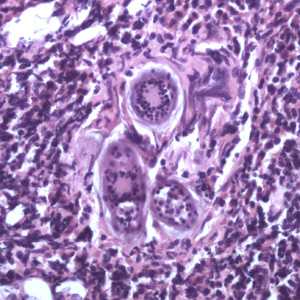
Figure E: Eggs of S. haematobium in a urinary bladder biopsy specimen, stained with H&E. Images courtesy of the Michael E. DeBakey V. A. Medical Center, Houston, TX.
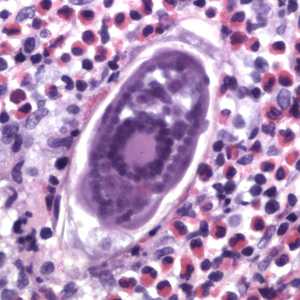
Figure F: Egg of S. haematobium in a urinary bladder biopsy specimen, stained with H&E. Images courtesy of the Michael E. DeBakey V. A. Medical Center, Houston, TX.
Schistosoma mansoni adults.
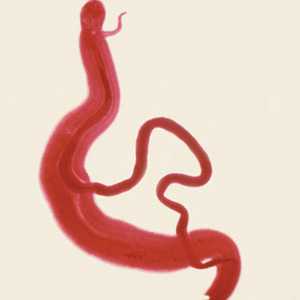
Figure A: Adults of S. mansoni. The thin female resides in the gynecophoral canal of the thicker male.
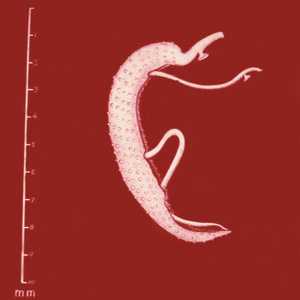
Figure B: Adults of S. mansoni. The thin female resides in the gynecophoral canal of the thicker male. Note the tuberculate exterior of the male.
Cross-sections of human tissues with Schistosoma spp. adults.
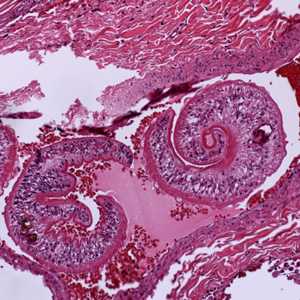
Figure A: Adults of Schistosoma sp. in lung tissue, stained with H&E. Image courtesy of Harvard Medical School, Cambridge, MA.
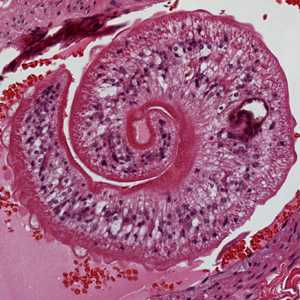
Figure B: Higher magnification of one of the worms in Figure A, showing the tuberculate exterior of the adult worm.
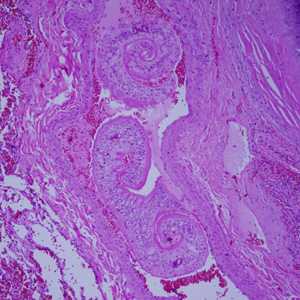
Figure C: Adults of Schistosoma spp. in lung tissue, stained with H&E. Images courtesy of Harvard Medical School, Cambridge, MA.
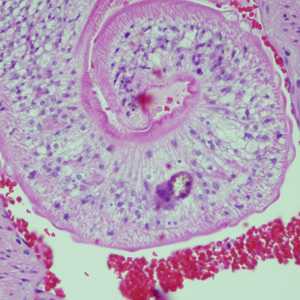
Figure D: Adults of Schistosoma spp. in lung tissue, stained with H&E. Images courtesy of Harvard Medical School, Cambridge, MA.
Intermediate hosts for Schistosoma spp.

Figure A: Biomphalaria sp., the intermediate host for S. mansoni..
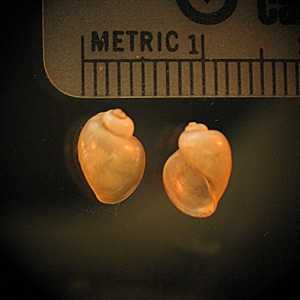
Figure B: Bulinus sp., the intermediate host for S. haematobium and S. intercalatum.
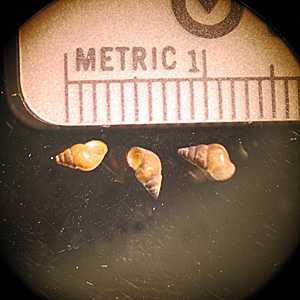
Figure C: Oncomelania sp., the intermediate host for S. japonicum.
Laboratory Diagnosis
Microscopic identification of eggs in stool or urine is the most practical method for diagnosis. Stool examination should be performed when infection with S. mansoni or S. japonicum is suspected, and urine examination should be performed if S. haematobium is suspected. Eggs can be present in the stool in infections with all Schistosoma species. The examination can be performed on a simple smear (1 to 2 mg of fecal material). Since eggs may be passed intermittently or in small amounts, their detection will be enhanced by repeated examinations and/or concentration procedures (such as the formalin-ethyl acetate technique). In addition, for field surveys and investigational purposes, the egg output can be quantified by using the Kato-Katz technique (20 to 50 mg of fecal material) or the Ritchie technique. Eggs can be found in the urine in infections with S. haematobium (recommended time for collection: between noon and 3 PM) and with S. japonicum. Detection will be enhanced by centrifugation and examination of the sediment. Quantification is possible by using filtration through a Nucleopore® membrane of a standard volume of urine followed by egg counts on the membrane. Tissue biopsy (rectal biopsy for all species and biopsy of the bladder for S. haematobium) may demonstrate eggs when stool or urine examinations are negative.
Antibody detection
Antibody detection can be useful to indicate schistosome infection in patients who have traveled in schistosomiasis endemic areas and in whom eggs cannot be demonstrated in fecal or urine specimens. Test sensitivity and specificity vary widely among the many tests reported for the serologic diagnosis of schistosomiasis and are dependent on both the type of antigen preparations used (crude, purified, adult worm, egg, cercarial) and the test procedure.
At CDC, a combination of tests with purified adult worm antigens are used for antibody detection. All serum specimens are tested by FAST-ELISA using Schistosoma mansoni adult microsomal antigen (MAMA). A positive reaction (greater than 9 units/µl serum) indicates infection with Schistosoma species. Sensitivity for S. mansoni infection is 99%, 95% for Schistosoma haematobium infection, and <50% for Schistosoma japonicuminfection. Specificity of this assay for detecting schistosome infection is 99%. Because test sensitivity with the FAST-ELISA is reduced for species other than S. mansoni, immunoblots of the species appropriate to the patient's travel history are also tested to ensure detection of S. haematobium and S. japonicum infections. Immunoblots with adult worm microsomal antigens are species-specific and so a positive reaction indicates the infecting species. The presence of antibody is indicative only of schistosome infection at some time and cannot be correlated with clinical status, worm burden, egg production, or prognosis. When submitting specimens, please include the patient's travel history so the appropriate Schistosoma species will be tested by immunoblot.
Reference:
Tsang VC, Wilkins PP. Immunodiagnosis of schistosomiasis. Immunol Invest 1997;26:175-188.
More on: Morphologic comparison with other intestinal parasites
Treatment Information
Infections with all major Schistosoma species can be treated with praziquantel. The timing of treatment is important since praziquantel is most effective against the adult worm and requires the presence of a mature antibody response to the parasite. For travelers, treatment should be at least 6-8 weeks after last exposure to potentially contaminated freshwater. One study has suggested an effect of praziquantel on schistosome eggs lodged in tissues. Limited evidence of parasite resistance to praziquantel has been reported based on low cure rates in recently exposed or heavily infected populations; however, widespread clinical resistance has not occurred. Thus, praziquantel remains the drug of choice for treatment of schistosomiasis. Host immune response differences may impact individual response to treatment with praziquantel. Although a single course of treatment is usually curative, the immune response in lightly infected patients may be less robust, and repeat treatment may be needed after 2 to 4 weeks to increase effectiveness. If the pre-treatment stool or urine examination was positive for schistosome eggs, follow up examination at 1 to 2 months post-treatment is suggested to help confirm successful cure.
| Schistosoma species infection | Praziquantel dose and Duration |
|---|---|
| Schistosoma mansoni, S. haematobium, S. intercalatum | 40 mg/kg per day orally in two divided doses for one day |
| S. japonicum, S. mekongi | 60 mg/kg per day orally in three divided doses for one day |
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
Note on Treatment During Lactation
Note on Treatment in Pediatric Patients
There is a lack of safety trial data for the use of praziquantel in children less than 4 years of age or pregnant women. However, this drug has been distributed widely in mass drug administration programs and WHO now recommends that pregnant women should be treated as part of those campaigns based on extensive experience with the drug and review of the veterinary and human evidence. Similarly, WHO reports that there is growing evidence that infected children as young as 1 year old can be effectively treated with praziquantel without serious side effects; however, the drug is commonly available in the form of large, hard-to-swallow pills, which puts young children at risk for choking and other difficulties swallowing the drug.
There is a lack of safety trial data for the use of praziquantel in children less than 4 years of age or pregnant women. However, this drug has been distributed widely in mass drug administration programs and WHO now recommends that pregnant women should be treated as part of those campaigns based on extensive experience with the drug and review of the veterinary and human evidence. Similarly, WHO reports that there is growing evidence that infected children as young as 1 year old can be effectively treated with praziquantel without serious side effects; however, the drug is commonly available in the form of large, hard-to-swallow pills, which puts young children at risk for choking and other difficulties swallowing the drug.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir