
Toxocariasis
[Toxocara canis] [Toxocara cati]
Causal Agents
Toxocariasis is caused by larvae of Toxocara canis (dog roundworm) and less frequently of T. cati (cat roundworm), two nematode parasites of animals.
Life Cycle

Toxocara canis accomplishes its life cycle in dogs, with humans acquiring the infection as accidental hosts. Unembryonated eggs are shed in the feces of the definitive host  . Eggs embryonate and become infective in the environment
. Eggs embryonate and become infective in the environment  . Following ingestion by dogs
. Following ingestion by dogs  , the infective eggs hatch and larvae penetrate the gut wall. In younger dogs, the larvae migrate through the lungs, bronchial tree, and esophagus; adult worms develop and oviposit in the small intestine
, the infective eggs hatch and larvae penetrate the gut wall. In younger dogs, the larvae migrate through the lungs, bronchial tree, and esophagus; adult worms develop and oviposit in the small intestine  . In older dogs, patent infections can also occur, but larval encystment in tissues is more common. Encysted stages are reactivated in female dogs during late pregnancy and infect by the transplacental and transmammary routes the puppies
. In older dogs, patent infections can also occur, but larval encystment in tissues is more common. Encysted stages are reactivated in female dogs during late pregnancy and infect by the transplacental and transmammary routes the puppies  , in whose small intestine adult worms become established
, in whose small intestine adult worms become established  . Puppies are a major source of environmental egg contamination. Toxocara canis can also be transmitted through ingestion of paratenic hosts: eggs ingested by small mammals (e.g. rabbits) hatch and larvae penetrate the gut wall and migrate into various tissues where they encyst
. Puppies are a major source of environmental egg contamination. Toxocara canis can also be transmitted through ingestion of paratenic hosts: eggs ingested by small mammals (e.g. rabbits) hatch and larvae penetrate the gut wall and migrate into various tissues where they encyst  . The life cycle is completed when dogs eat these hosts
. The life cycle is completed when dogs eat these hosts  and the larvae develop into egg-laying adult worms in the small intestine. Humans are accidental hosts who become infected by ingesting infective eggs in contaminated soil
and the larvae develop into egg-laying adult worms in the small intestine. Humans are accidental hosts who become infected by ingesting infective eggs in contaminated soil  or infected paratenic hosts
or infected paratenic hosts 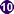 . After ingestion, the eggs hatch and larvae penetrate the intestinal wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, brain, muscle, eyes)
. After ingestion, the eggs hatch and larvae penetrate the intestinal wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, brain, muscle, eyes)  . While the larvae do not undergo any further development in these sites, they can cause severe local reactions that are the basis of toxocariasis. The two main clinical presentations of toxocariasis are visceral larva migrans and ocular larva migrans. Diagnosis is usually made by serology or the finding of larvae in biopsy or autopsy specimens.
. While the larvae do not undergo any further development in these sites, they can cause severe local reactions that are the basis of toxocariasis. The two main clinical presentations of toxocariasis are visceral larva migrans and ocular larva migrans. Diagnosis is usually made by serology or the finding of larvae in biopsy or autopsy specimens.
Geographic Distribution
Worldwide.
Clinical Presentation
Many human infections are asymptomatic, with only eosinophilia and positive serology. The two main clinical presentations of toxocariasis are visceral larva migrans (VLM) and ocular larva migrans (OLM). In VLM, which occurs mostly in preschool children, the larvae invade multiple tissues (liver, heart, lungs, brain, muscle) and cause various symptoms including fever, anorexia, weight loss, cough, wheezing, rashes, hepatosplenomegaly, and hypereosinophilia. Death can occur rarely, by severe cardiac, pulmonary or neurologic involvement. In OLM, the larvae produce various ophthalmologic lesions, which in some cases have been misdiagnosed as retinoblastoma, resulting in surgical enucleation. OLM often occurs in older children or young adults, with only rare eosinophilia or visceral manifestations.
Toxocara sp. eggs.
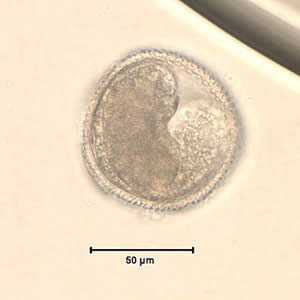
Figure A: Toxocara sp. egg teased from an adult worm. The worm was never identified, but the egg size is most consistent with T. cati. Image courtesy of the New Jersey State Public Health Laboratory.
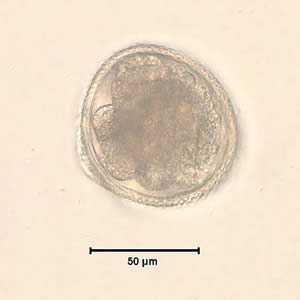
Figure B: Toxocara sp. egg teased from an adult worm. The worm was never identified, but the egg size is most consistent with T. cati. Image courtesy of the New Jersey State Public Health Laboratory.
Figure C: Toxocara sp. eggs teased from an adult worm and stained with iodine, magnification at 100×. The adult worm was never identified in this case. Image courtesy of the Alaska State Public Health Laboratory.
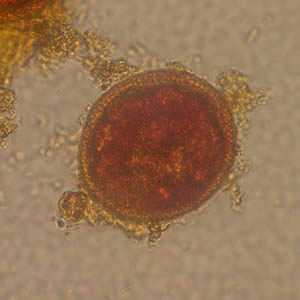
Figure D: Egg from same specimen as C but at 400× magnification. Object measured approximately 75 micrometers.
Toxocara canis larva hatching.
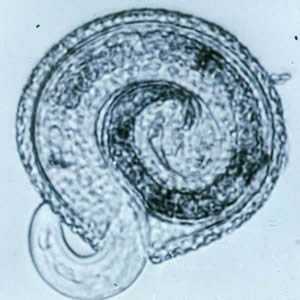
Figure A: Toxocara canis larva beginning to hatch.
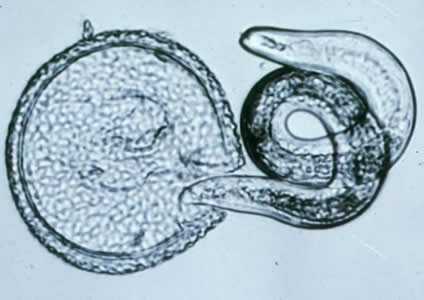
Figure B: T. canis larva hatching.
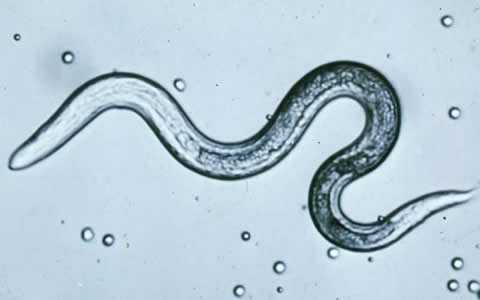
Figure C: T. canis larva.
Adult Toxocara sp. worms.
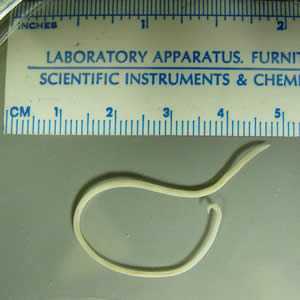
Figure A: Toxocara sp. adult female. Stretched out, the worm measured 7.5 cm. Image courtesy of the Alaska State Public Health Laboratory.
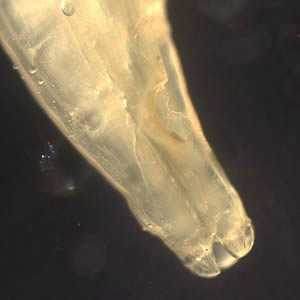
Figure B: Close-up of the anterior end of Toxocara sp., showing the three lips characteristic of ascarid worms. Image courtesy of the New Jersey State Public Health Laboratory.

Figure C: Close-up of the anterior end of Toxocara cati, showing the three lips characteristic of ascarid worms.
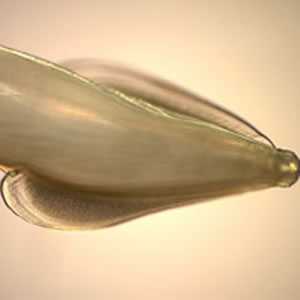
Figure D: Side view of Image C, showing the broad, arrow-shaped alae with striations, characteristic of T. cati.

Figure E:Close-up of the posterior end of T. cati, showing a prominent point at the end of the “tail.”

Figure F: Close-up of the posterior end of Toxocara sp. Image courtesy of the New Jersey State Public Health Laboratories.
Toxocara sp. in tissue sections stained with hematoxylin and eosin.
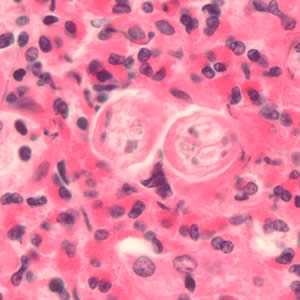
Figure A: Cross-section of Toxocara sp. larvae in liver tissue stained with hematoxylin and eosin (H&E).
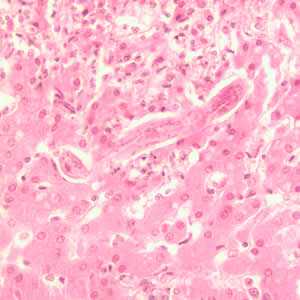
Figure B: Longitudinal section of a Toxocara sp. larva in liver tissue stained with H&E.
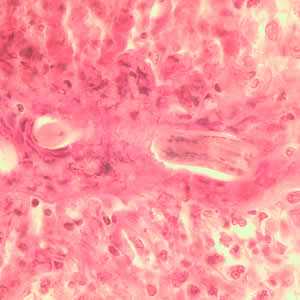
Figure C: Longitudinal section of a Toxocara sp. larva in lung tissue stained with H&E.
Laboratory Diagnosis
In this parasitic disease the diagnosis does not rest on identification of the parasite. Since the larvae do not develop into adults in humans, a stool examination would not detect any Toxocara eggs. However, the presence of Ascaris and Trichuris eggs in feces, indicating fecal exposure, increases the probability of Toxocara in the tissues. For both VLM and OLM, a presumptive diagnosis rests on clinical signs, history of exposure to puppies, laboratory findings (including eosinophilia), and the detection of antibodies to Toxocara.
Antibody Detection
Antibody detection tests are the only means of confirmation of a clinical diagnosis of visceral larva migrans (VLM), ocular larva migrans (OLM), and covert toxocariasis (CT), the most common clinical syndromes associated with Toxocara infections. The currently recommended serologic test for toxocariasis is enzyme immunoassay (EIA) with larval stage antigens extracted from embryonated eggs or released in vitro by cultured infective larvae. The latter, Toxocara excretory-secretory (TES) antigens, are preferable to larval extracts because they are convenient to produce and because an absorption-purification step is not required for obtaining maximum specificity. Evaluation of the true sensitivity and specificity of serologic tests for toxocariasis in human populations is not possible because of the lack of parasitologic methods to detect Toxocara parasites. These inherent problems result in underestimations of sensitivity and specificity. Evaluation of the Toxocara EIA in groups of patients with presumptive diagnoses of VLM or OLM indicated sensitivity of 78% and 73%, respectively, at a titer of >1:32. When the cutoff titer for OLM cases was lowered to 1:8, sensitivity was increased to 90%. Further confirmation of the specificity of the serologic diagnosis of OLM can be obtained by testing aqueous or vitreous humor samples for antibodies. Specificity has been reported to be >90% at a titer of >1:32. When interpreting the serologic findings, clinicians must be aware that a measurable titer does not necessarily indicate current clinical Toxocara canis infection. In most human populations, a small number of those tested have positive EIA titers that apparently reflect the prevalence of asymptomatic toxocariasis. In the United States The age adjusted seroprevalence rate was estimated to be approximately 14%, using sera from over 20,000 participants in the Third National Health and Nutrition Examination Survey (1988–1994).
References:
- Won KY, Kruszon-Moran D, Schantz PM, and Jones JL. 2006. National Seroprevalence and Risk Factors for Zoonotic Toxocara spp. Infection. Am. J. Trop. Med. Hyg. 79: 552–557.
- Smith HV. Antibody reactivity in human toxocariasis. In: Lewis JW, Maizels RM, editors. Toxocara and toxocariasis: clinical, epidemiological, and molecular perspectives. London, UK: Institute of Biology and the British Society for Parasitology; 1993. p. 91-109.
Treatment Information
Treatment with albendazole or mebendazole is indicated for visceral toxocariasis, although optimal duration of treatment is undefined. Both drugs are metabolized in the liver; prolonged use of albendazole (weeks to months) has led to development of pancytopenia in some patients with compromised liver function. Patients on long term treatment should be monitored by serial blood cell counts. However, albendazole has been used to treat millions of patients worldwide and in mass drug administration campaigns, and it is considered to be a safe drug with low toxicity record. In addition to antiparasitic therapy, symptomatic therapy including steroid treatment to control inflammation may be indicated.
| Drug | Dose and duration |
|---|---|
| Albendazole | 400 mg by mouth twice a day for five days (both adult and pediatric dosage) |
| Mebendazole | 100-200 mg by mouth twice a day for five days (both adult and pediatric dosage) |
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
For ocular toxocariasis, the goal of treatment is to minimize damage to the eye. Systemic antiparasitic treatment with albendazole or mebendazole at the same doses as for visceral disease may be beneficial for active disease. Attempts to surgically remove the larva may be unsuccessful. Control of inflammation in the eye by use of topical or systemic steroids may be indicated. For patients with quiescent disease, improved outcomes may result from surgical intervention to prevent further damage due to chronic inflammation.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir