
Trichinellosis
[Trichinella spp.]
Causal Agents
Trichinellosis (trichinosis) is caused by nematodes (roundworms) of the genus Trichinella. In addition to the classical agent T. spiralis (found worldwide in many carnivorous and omnivorous animals), several other species of Trichinella are now recognized, including T. pseudospiralis (mammals and birds worldwide), T. nativa (Arctic bears), T. nelsoni (African predators and scavengers), T. britovi (carnivores of Europe and western Asia), and T. papuae (wild and domestic pigs, Papua New Guinea and Thailand). Trichinella zimbabwensis is found in crocodiles in Africa but to date there are no known associations of this species with human disease.
Life Cycle

Depending on the classification used, there are several species of Trichinella: T. spiralis, T. pseudospiralis, T. nativa, T. murelli, T. nelsoni, T. britovi, T. papuae, and T. zimbabwensis, all but the last of which have been implicated in human disease. Adult worms and encysted larvae develop within a single vertebrate host, and an infected animal serves as a definitive host and potential intermediate host. A second host is required to perpetuate the life cycle of Trichinella. The domestic cycle most often involved pigs and anthropophilic rodents, but other domestic animals such as horses can be involved. In the sylvatic cycle, the range of infected animals is great, but animals most often associated as sources of human infection are bear, moose and wild boar.
Trichinellosis is caused by the ingestion of undercooked meat containing encysted larvae (except for T. pseudospiralis and T. papuae, which do not encyst) of Trichinella species  . After exposure to gastric acid and pepsin, the larvae are released from the cysts
. After exposure to gastric acid and pepsin, the larvae are released from the cysts  and invade the small bowel mucosa where they develop into adult worms
and invade the small bowel mucosa where they develop into adult worms  . Females are 2.2 mm in length; males 1.2 mm. The life span in the small bowel is about four weeks. After 1 week, the females release larvae
. Females are 2.2 mm in length; males 1.2 mm. The life span in the small bowel is about four weeks. After 1 week, the females release larvae  that migrate to striated muscles where they encyst
that migrate to striated muscles where they encyst 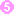 . Diagnosis is usually made based on clinical symptoms, and is confirmed by serology or identification of encysted or non-encysted larvae in biopsy or autopsy specimens.
. Diagnosis is usually made based on clinical symptoms, and is confirmed by serology or identification of encysted or non-encysted larvae in biopsy or autopsy specimens.
Geographic Distribution
Worldwide. Most common in parts of Europe and the United States.
Clinical Presentation
Light infections may be asymptomatic. Intestinal invasion can be accompanied by gastrointestinal symptoms (diarrhea, abdominal pain, vomiting). Larval migration into muscle tissues (one week after infection) can cause periorbital and facial edema, conjunctivitis, fever, myalgias, splinter hemorrhages, rashes, and peripheral eosinophilia. Occasional life-threatening manifestations include myocarditis, central nervous system involvement, and pneumonitis. Larval encystment in the muscles causes myalgia and weakness, followed by subsidence of symptoms.
Encysted larvae of Trichinella in tissue, stained with hematoxylin and eosin (H&E).
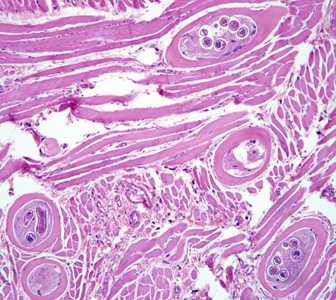
Figure A: Encysted larvae of Trichinella sp. in muscle tissue, stained with hematoxylin and eosin (H&E). The image magnification is 200x.
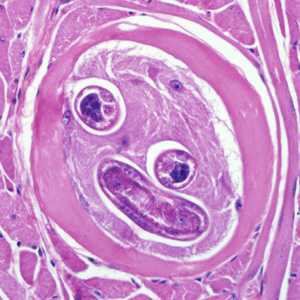
Figure B: Encysted larvae of Trichinella sp. in muscle tissue, stained with hematoxylin and eosin (H&E). The image magnification is 400x.
Figure C: Encysted larvae of Trichinella sp. in muscle tissue, stained with hematoxylin and eosin (H&E). Image was captured at 400x magnification.
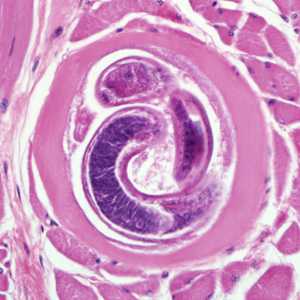
Figure D: Encysted larvae of Trichinella sp. in muscle tissue, stained with hematoxylin and eosin (H&E). Image was were captured at 400x magnification.
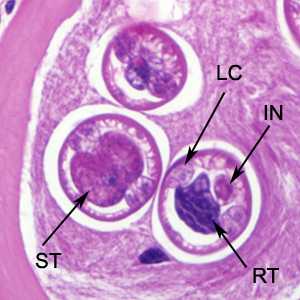
Figure E: Higher-magnification of the larvae in Figure C. Shown in these cuts are a nucleated stichocyte (ST), prominent lateral chords, or bacillary bands, (LC), immature reproductive tubes (RT), and the intestine (IN). Image captured at 1000x magnification.
Trichinella larvae in tongue tissue of a rat, stained with H&E.
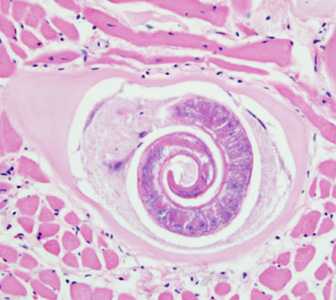
Figure A: Trichinella larva in tongue muscle of a rat, stained with hematoxylin and eosin (H&E). Image was captured at 400x magnification.
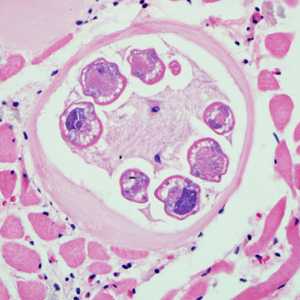
Figure B: Trichinella larva in tongue muscle of a rat, stained with hematoxylin and eosin (H&E). Image was captured at 400x magnification.
Larvae of Trichinella from bear meat.
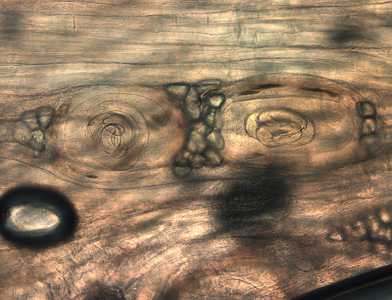
Figure A: Trichinella larvae in pressed bear meat, partially digested with pepsin.
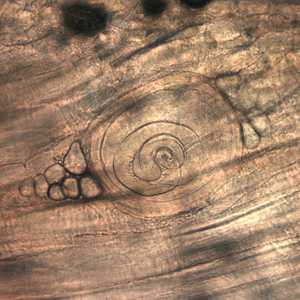
Figure B: Trichinella larva in pressed bear meat, partially digested with pepsin.

Figure C: Trichinella larvae in pressed bear meat, partially digested with pepsin.
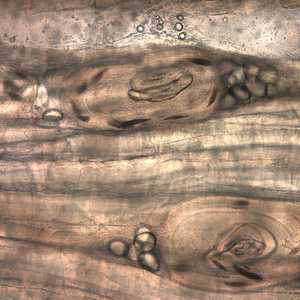
Figure D: Trichinella larvae in pressed bear meat, partially digested with pepsin.
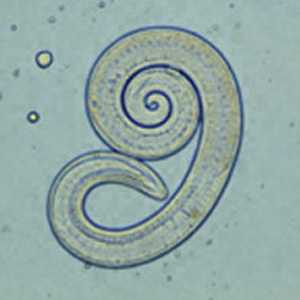
Figure E: Larva of Trichinella liberated from bear meat. This larva is from a different case than those shown in Figures A-D.

Figure F: Larva of Trichinella liberated from bear meat. This larva is from a different case than those shown in Figures A-D.
Laboratory Diagnosis
The suspicion of trichinellosis (trichinosis), based on history, clinical symptoms, and eosinophilia, can be confirmed by specific diagnostic tests, including antibody detection, muscle biopsy, and microscopy.
Antibody Detection
Immunodiagnostic tests currently available in the U.S. include enzyme immunoassays (EIA) that detect Trichinella-specific antibodies. EIAs utilize antigen preparations that may be crude extracts prepared from homogenates of T. spiralis muscle larvae or excretory-secretory (ES) products produced by cultured larvae. The TSL-1 group of larval secretory antigens are conserved in all species/isolates of Trichinella and thus can be used to detect infection in animals or people infected with any of the types of Trichinella currently recognized. Positive reactions are detectable at some time during infection in serum samples of 80% to 100% of patients with clinically symptomatic trichinellosis (trichinosis). Antibody levels are often not detectable until 3 to 5 weeks post-infection, well after the onset of acute-stage illness. Antibody development is also affected by the infecting dose of larvae: the higher the infecting dose, the faster the patient's antibody response will develop. Multiple serum specimens should be drawn several weeks apart to demonstrate seroconversion in patients whose initial specimen was negative. IgG, IgM, and IgE antibodies are detectable in many patients; however, tests based on IgG antibodies are most sensitive. Antibody levels peak in the second or third month post-infection and then decline slowly for several years. In our experience at CDC, EIAs with ES antigens detect antibodies earlier than bentonite flocculation (BF) in 25% of serum specimens from patients with acute infection, but EIAs also remain positive for longer periods after infection than the BF, and are reactive in a larger proportion of persons with no clinical evidence of trichinellosis.
Reference:
Murrell KD, Bruschi F. Clinical trichinellosis. Prog Clin Parasitol 1994;4:117-150.
Treatment Information
Prompt treatment with antiparasitic drugs can help prevent the progression of trichinellosis by killing the adult worms and so preventing further release of larvae. Once the larvae have become established in skeletal muscle cells, usually by 3 to 4 weeks post infection, treatment may not completely eliminate the infection and associated symptoms. Treatment with either mebendazole or albendazole is recommended. If treatment is not initiated within the first several days of infection, more prolonged or repeated courses of treatment may be necessary. Both drugs are considered relatively safe but have been associated with side effects including bone marrow suppression. Patients on longer courses of therapy should be monitored by serial complete blood counts to detect any adverse effects promptly and discontinue treatment. Albendazole and mebendazole are not approved for use in pregnant women or children under the age of 2 years. In addition to antiparasitic medication, treatment with steroids is sometimes required in more severe cases.
| Drug | Adult and pediatric dose |
|---|---|
| Albendazole | 400 mg twice a day by mouth for 8 to 14 days |
| Mebendazole | 200 to 400 mg three times a day by mouth for 3 days, then 400 to 500 mg three times a day by mouth for 10 days |
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir