Specimen Submission Form
Submitting Specimens to CDC
Submitters sending specimens to CDC for laboratory testing should supply all pertinent information associated with the specimen(s). This information will allow the laboratory to effectively review the test order and perform the appropriate test(s). The information supplied will be included in the laboratory report.
Specimens submitted for testing must be accompanied by CDC Form 50.34 [PDF – 2 pages].*
Features of the form:
- Pick-lists to select the correct form, order valid tests, enter accurate information
- Interactive Test Directory
- Easier data entry and printing using your computer
- Accurate data transfer using barcodes
- Download and save the form with your data
- To view the latest changes to the 50.34 Form, view the major list of changes here. [PDF – 3 pages]
If you have any questions about the form or the submission process, check the “Help and FAQs” section or the “Training” Section to view a training webinar on the new submission form and view form specific training manuals.
Troubleshooting the 50.34 Specimen Submission Form
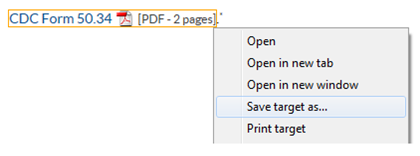 Trouble downloading the CDC Form 50.34? Try one of these options:
Trouble downloading the CDC Form 50.34? Try one of these options:
- Right click on the “CDC Form 50.34” PDF link. From the menu, select “Save Target As…” (NOTE: Depending on your browser, the wording may be different to save the file/link). Save the file as an Adobe document
- Try opening and downloading the form using another browser, such as Internet Explorer 11
- Make sure you have the latest version of Adobe Reader
* Persons with disabilities experiencing problems accessing this document should contact CDC-INFO by either completing the form at www.cdc.gov/cdc-info/requestform.html and use subject “508 Accommodation PR#31”, or by calling 800-232-4636 (TTY number: 888-232-6348) and ask for 508 Accommodation PR#31.
- Page last reviewed: July 12, 2017
- Page last updated: February 16, 2017
- Content source:


 ShareCompartir
ShareCompartir