Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
2009 Pandemic Influenza A (H1N1) in Pregnant Women Requiring Intensive Care --- New York City, 2009
Pregnant women are at increased risk for severe illness and complications from infection with seasonal influenza (1--3) and 2009 pandemic influenza A (H1N1) (4--6). To characterize the severity of 2009 H1N1 infection in pregnant women, the New York City Department of Health and Mental Hygiene (DOHMH) conducted active and passive surveillance for cases of 2009 H1N1 infection in pregnant women requiring intensive care. This report summarizes the results of that surveillance, which found that, during 2009, 16 pregnant women and one who was postpartum were admitted to New York City intensive-care units (ICUs). Two women died. Of the 17 women, 12 had no recognized risk factors for severe influenza complications other than pregnancy (7). All 17 women received antiviral treatment with oseltamivir; however, treatment was initiated ≤2 days after symptom onset in only one woman and was begun ≥5 days after symptom onset in four women. Because initiation of antiviral treatment ≤2 days after onset is associated with better outcomes (5,6), pregnant women should be encouraged to seek medical care immediately if they develop influenza-like symptoms, and health-care providers should initiate empiric antiviral therapy for these women as soon as possible, even if >2 days after symptom onset. Health departments and health-care providers should educate pregnant and postpartum women regarding the risks posed by influenza and highlight the effectiveness and safety of influenza vaccination. Obstetricians and other health-care providers should offer influenza vaccination to their pregnant patients.
To identify cases of 2009 H1N1 infection in pregnant and postpartum women, beginning April 25, 2009, DOHMH conducted surveillance for hospitalizations and deaths during three separate periods. Surveillance methods varied as the 2009 H1N1 pandemic evolved and influenza activity changed in New York City. During April--June, DOHMH conducted citywide active surveillance for deaths from 2009 H1N1 and enhanced citywide surveillance for hospitalized cases of influenza in pregnant and postpartum women, actively requesting specimens and testing for 2009 H1N1 at the New York City Public Health Laboratory. During July--September, influenza activity was low in New York City; however, ongoing passive surveillance was conducted for hospitalized patients who tested positive for influenza A. During October--December, citywide surveillance was passive, except active surveillance was reestablished at five sentinel hospitals. During all three periods, data on pregnancy, ICU status, and vital status were collected for all patients hospitalized with 2009 H1N1 throughout New York City. Chart abstractions for all identified cases were conducted by medical epidemiologists at DOHMH. For this case series, a case was defined as severe illness with laboratory-confirmed or probable 2009 H1N1 infection* in a woman who was pregnant or postpartum (within 6 weeks of delivery), resulting in admission to an ICU or death.
During 2009, a total of 17 patients (16 pregnant women and one who was postpartum) met the case definition; nine were admitted to ICUs during April--June, and eight were admitted during October--December. No patients met the case definition during July--September. Median age of the patients was 23 years (range: 20--37 years), and median gestational age at hospital admission was 34 weeks (range: 6--41 weeks) (Table). Median length of hospital stay was 12 days (range: 4--38 days). Five of the 17 women had risk factors for severe influenza complications recognized by the Advisory Committee for Immunization Practices (ACIP) other than pregnancy (7). One patient had asthma and cardiovascular disease (diagnosed postmortem). The other four patients had sickle cell disease, asthma, seizure disorder, and diabetes mellitus, respectively. Only one of the 17 patients had received 2009 H1N1 vaccine, according to the medical records; she had been administered H1N1 vaccine >4 weeks before hospitalization, after being administered seasonal influenza vaccine >8 weeks before hospitalization. Eleven of the 17 women were in their third trimester, including five who developed acute respiratory distress syndrome (ARDS). All 17 women received antiviral treatment with oseltamivir; however, treatment was initiated ≤2 days after symptom onset in only one woman and was begun ≥5 days after symptom onset in four women; initiation of antiviral treatment ≤2 days after onset is associated with better outcomes (5,6).
Four of the nine women who gave birth during their 2009 H1N1 hospitalization had an emergency cesarean delivery; eight infants were live-born (including one who died soon after birth), and one was stillborn. Six of the eight live-born infants were admitted to a neonatal ICU.
Illustrative Case Reports
Patient 1. A woman aged 27 years who was at 32 weeks' gestation (Table) went to her primary care physician during May 2009 after 1 day of fever and cough (Figure). She was treated with antibiotics for 3 days without improvement. Five days after symptom onset, she went to the emergency department, reporting persistent fevers, chills, cough, wheezing, and an episode of near-syncope. On admission she was afebrile, with a respiratory rate of 22 breaths per minute, a heart rate of 96 beats per minute, blood pressure of 100/70 mmHg, and oxygen saturation of 99% on room air. A chest radiograph revealed bilateral lobar pneumonia, and she was treated for community-acquired pneumonia. On hospital day 2, she developed fever to 102.9°F (39.4°C), tachycardia (141 beats per minute) and severe respiratory distress. ARDS was diagnosed, and the patient was transferred to the ICU for mechanical ventilation and treated empirically with oseltamivir, 75 mg twice daily. Rapid influenza diagnostic tests performed on nasopharyngeal specimens 1 day before hospital admission and on hospital day 3 were negative for influenza.
On hospital day 4, because her oxygen saturations worsened to approximately 75% despite maximal ventilation settings, an emergency cesarean delivery was performed. During the procedure, the patient was hypotensive and required multiple blood transfusions. Cultures from bronchoalveolar lavage collected the previous day grew Acinetobacter baumanii. On hospital day 11, diagnosis of 2009 H1N1 was confirmed from a nasopharyngeal swab specimen submitted to the DOHMH Public Health Laboratory on hospital day 3. On hospital day 16, because of refractory hypoxemia and severe ARDS, the woman was transferred to another hospital ICU for extracorporeal membrane oxygenation (ECMO), and oseltamivir was increased to 150 mg, twice daily. Her subsequent hospital course was complicated by volume overload, septic shock, and ventilator-associated pneumonia with Klebsiella pneumoniae and A. baumanii. She died on hospital day 38, a total of 42 days after symptom onset (Figure). At birth, her infant weighed 1,500 g and had Apgar scores of 1 at 1 minute and 1 at 5 minutes after birth; the infant stopped breathing, and neonatal resuscitation efforts were unsuccessful.
Patient 15. A woman aged 21 years who was at 34 weeks' gestation was admitted to a hospital during November 2009 (Table) with respiratory distress; 6 days of fever, cough, and myalgia; and 2 days of blood-tinged sputum (Figure). A few days before admission she had been prescribed antibiotics and oseltamivir by her primary-care provider but only took the antibiotics. On admission, she had a fever of 100.9°F (38.3°C), tachycardia (141 beats per minute), blood pressure of 101/66 mmHg, and a respiratory rate of 20 breaths per minute; her chest radiograph showed bilateral pulmonary infiltrates consistent with ARDS. On hospital day 2, she was transferred to the ICU for mechanical ventilation; she developed septic shock requiring vasopressors and was treated with broad-spectrum antibiotics and oseltamivir, 150 mg twice daily. Her respiratory status deteriorated and she underwent emergency cesarean delivery.
On hospital day 3, the patient was transferred to another hospital ICU for ECMO treatment of severe ARDS and septic shock. Soon after transfer, she experienced cardiac arrest with ventricular fibrillation; defibrillation was successful after less than 2 minutes of no pulse. Oseltamivir was changed to empiric intravenous peramivir and broad-spectrum antibiotics. On hospital day 4, diagnosis of 2009 H1N1 was confirmed from a nasopharyngeal swab specimen submitted to the DOHMH Public Health Laboratory on hospital day 2. Her hospital course included spontaneous pneumothoraces, hypotension requiring vasopressors, an episode of asystole, infection with K. pneumoniae, fevers to 107.1°F (41.7°C), disseminated intravascular coagulation, and tracheostomy placement. Her respiratory status improved, and ECMO was discontinued on hospital day 14. She was transferred from the ICU without supplemental oxygen on hospital day 26 and discharged home with physical therapy on hospital day 32. On discharge she was fully alert and walking with assistance. At birth, her infant weighed 2,080 grams and had Apgar scores of 3 at 1 minute and 6 at 5 minutes after birth. The infant required mechanical ventilation and was treated with antibiotics for suspected sepsis; oseltamivir was not administered. The infant improved and was discharged on day 3 of life.
Reported by
A Fine, MD, C Dentinger, MS, TF Johnson, MD, A Kossowski, MD, L Steiner-Sichel, MPH, AG Schwarz, MPH, New York City Dept of Health and Mental Hygiene. LK Hartman, MD, Oak Ridge Institute for Science and Education, Oak Ridge, Tennessee. MA Honein, PhD, National Center on Birth Defects and Developmental Disabilities; D Jamieson, MD, National Center for Chronic Disease Prevention and Health Promotion; T Uyeki, MD, National Center for Immunization and Respiratory Diseases; T Al-Samarrai, MD, AA Creanga, MD, PhD, SB Graitcer, MD, EIS officers, CDC.
Editorial Note
An analysis of New York City 2009 H1N1 hospitalizations during May--June 2009 showed that pregnant women were 7.2 times more likely to be hospitalized and 4.3 times more likely to be admitted to an ICU than nonpregnant women (6). Immunologic changes, increased ventilatory demand, and decreased functional residual capacity and oncotic pressure all are postulated to predispose pregnant and postpartum women to severe respiratory complications from influenza virus infection (5,6).
The case series in this report highlights some delays in pregnant women seeking care and obtaining appropriate diagnosis and treatment of 2009 H1N1 virus infection in New York City, despite extensive outreach to the public and health-care providers by public health officials. The illustrative cases highlight some factors contributing to the delays, including false-negative rapid diagnostic test results and not taking oseltamivir as prescribed. In addition, only one of the 17 women was reported to have received 2009 H1N1 vaccine. Although no vaccine is 100% effective, vaccination remains the most important and effective means of preventing influenza among pregnant women.
The findings in this report are subject to at least three limitations. First, this report represents a case series rather than a population-based study, and methods of case ascertainment and influenza activity in New York City differed among the April--June, July-September, and October--December periods. The number of severe illnesses from 2009 H1N1 infection in pregnant women identified during these different periods might not be comparable for various reasons. For example, the threshold for admission to an ICU might have been lower in the fall than in the spring, given increased awareness of the potential severity of 2009 H1N1 infection in pregnant women. Second, underascertainment of cases might have occurred during all three periods because of limitations in active case-finding. Finally, 2009 H1N1 vaccine was not available until October, and the vaccination status for most of the 17 women was unknown; therefore, no conclusions can be drawn regarding the prevalence of vaccination in this group.
All clinicians, including obstetricians and health-care providers, should maintain a high index of suspicion for influenza when surveillance data suggest that influenza is circulating in a community. Pregnant and postpartum patients should be educated to recognize influenza-like symptoms and counseled to seek care immediately and to take antiviral therapy as prescribed (9). Health-care providers should ensure prompt evaluation and early empiric treatment with oseltamivir, irrespective of negative rapid influenza diagnostic test results; treatment with antipyretics and antibiotics also should be considered when indicated (5,8,10).
For pregnant or postpartum women and for those women considering becoming pregnant, clinicians and health departments should emphasize the importance of vaccination against seasonal influenza and 2009 H1N1 to prevent life-threatening complications. Although 2009 H1N1 activity has declined in the United States, the virus is still circulating and causing illness, and increases in influenza activity remain possible. Clinicians caring for pregnant and postpartum women should continue to encourage influenza vaccination during this and subsequent years and remember the importance of prompt empiric antiviral therapy for pregnant or postpartum patients with possible 2009 H1N1 influenza.
Acknowledgments
The findings in this report are based, in part, on contributions by S Balter, MD, S Beatrice, PhD, J Fu, PhD, LE Jones, E Lee, MD, W Oleszko, PhD, JE Sackoff, PhD, E Westheimer, MSc, New York City Dept of Health and Mental Hygiene; S Chu, PhD, C Shapiro-Mendoza, PhD, and K MacFarlane, MPH, National Center for Chronic Disease Prevention and Health Promotion, CDC.
References
- Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 2007;176:463--8.
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959;78:1172--5.
- Harris JW. Influenza occurring in pregnant women. JAMA 1919;72:978--80.
- Jamieson DJ, Honein MA, Rasmussen SA, et al; Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451--8.
- Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med 2009; 362:27--35.
- Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women: New York City, May--June 2009. Obstet Gynecol 2010;115:717--26.
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8).
- CDC. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus---United States, 2009. MMWR 2009;58:826--9.
- The American College of Obstetricians and Gynecologists. 2009--2010 influenza season assessment and treatment for pregnant women with influenza-like illness. Washington, DC: The American College of Obstetricians and Gynecologists; October 15, 2009. Available at http://www.acog.org/departments/resourcecenter/2009h1n1triagetreatment.pdf. Accessed March 19, 2010.
- CDC. Updated interim recommendations for obstetric health care providers related to use of antiviral medications in the treatment and prevention of influenza for the 2009--2010 season. December 29, 2009. Available at http://www.cdc.gov/h1n1flu/pregnancy/antiviral_messages.htm. Accessed March 19, 2010.
* In 15 of the cases, 2009 H1N1 was confirmed by real-time reverse transcription--polymerase chain reaction. Two cases with laboratory evidence of influenza A that were not subtyped were considered probable 2009 H1N1 because surveillance data indicated >90% of circulating influenza A in New York City at the time was 2009 H1N1.
What is already known on this topic?
Pregnant women have an increased risk for severe illness and complications from seasonal and pandemic influenza virus infection.
What is added by this report?
During 2009 in New York City, among 17 pregnant or postpartum women who were admitted to intensive-care units with severe illness from 2009 H1N1 influenza virus infection, two maternal deaths, one infant death, and a stillbirth resulted; for some of these patients, delays in care-seeking, diagnosis, and treatment of influenza might have increased the potential for rapid clinical decline.
What are the implications for public health practice?
Health departments and health-care providers should educate pregnant and postpartum women to recognize influenza-like symptoms and seek care promptly, and emphasize the need for prompt empiric antiviral treatment when influenza is circulating in the community; obstetricians and other health-care providers should offer influenza vaccination to their pregnant patients.
FIGURE. Timeline of key events in two cases of severe 2009 pandemic influenza A (H1N1) in pregnant women hospitalized in intensive-care units --- New York City, 2009
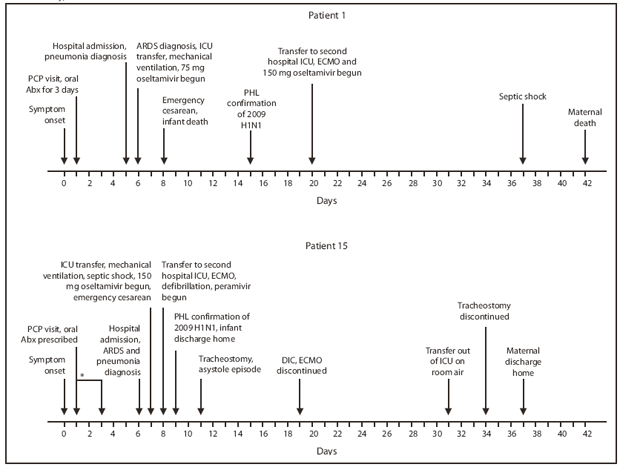
Abbreviations: PCP = primary-care provider, Abx = antibiotics, ICU = intensive-care unit, ARDS = acute respiratory distress syndrome, PHL = New York City Department of Health and Mental Hygiene Public Health Laboratory, ECMO = extracorporeal membrane oxygenation, DIC = disseminated intravascular coagulation.
* Date of PCP visit was not confirmed but was 1--3 days after symptom onset.
Alternate Text: The figure above shows two timelines of key events in the treatment cases of two pregnant women (patient 1 and patient 15) who were hospitalized in New York City intensive care units with 2009 pandemic influenza A (H1N1) during 2009.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


