FIGURE. Coverage with ≥1 dose of rotavirus vaccine (RV) among infants aged 5 months --- Immunization Information System (IIS) sentinel sites, June 2006--June 2009*
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Rotavirus Vaccination Coverage Among Infants Aged 5 Months --- Immunization Information System Sentinel Sites, United States, June 2006--June 2009
In February 2006, the Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination of all U.S. infants with 3 doses of a pentavalent rotavirus vaccine administered at ages 2, 4, and 6 months (1). In June 2008, ACIP updated its recommendations to include use of a second rotavirus vaccine, a 2-dose monovalent vaccine, administered at ages 2 and 4 months (1). The maximum age for the first dose of either rotavirus vaccine (RV) is 14 weeks and 6 days. CDC recently analyzed data from Immunization Information System (IIS) sentinel sites 1) to assess trends in coverage with ≥1 dose of RV during June 2006--June 2009 among infants aged 5 months and 2) to compare RV coverage in the second quarter of 2009 with that of two other routinely-recommended vaccines for U.S. infants: diphtheria, tetanus, and acellular pertussis (DTaP) vaccine, and 7-valent pneumococcal conjugate vaccine (PCV7). RV coverage increased following vaccine introduction and, in June 2009, averaged 72% at the eight currently participating IIS sentinel sites. However, ≥1 dose RV coverage among infants aged 5 months was 13% lower than the average coverage with ≥1 dose of DTaP and PCV7 at these same sites. Lower RV coverage could reflect typical new-vaccine coverage dynamics, the presence of RV-specific barriers (2,3), or both. Identifying and reducing barriers to vaccination and educating parents and providers about the health benefits of rotavirus vaccination should increase coverage and help prevent severe rotavirus disease.
IIS sentinel sites are a subset of IIS* that receive additional CDC support to promote improved data quality, functionality, and timeliness. Sentinel sites have high health-care provider participation (>85%), child enrollment (>85% of children aged <19 years), and timely capture of administered vaccines (>70% of doses are reported to the IIS within 30 days of vaccination). Although not designed to be representative of the U.S. population, IIS sentinel sites are population based and cover more than 1.8 million children aged <6 years residing in diverse regions of the United States. The eight IIS sentinel sites participating in the current 2008--2012 project cycle are located in Arizona, Colorado, Michigan, Minnesota, New York City, North Dakota, Oregon, and Wisconsin.† Four of these sites (Arizona, Michigan, Minnesota, and Oregon) also participated in the 2004--2007 project cycle.
To assess trends in RV coverage, CDC calculated site-specific coverage with ≥1 dose of RV among infants aged 5 months for each quarter of the evaluation period (second quarter 2006--second quarter 2009) for each of the four IIS participating as sentinel sites during both the 2004--2007 and the current (2008--2012) project cycles. Site-specific coverage was calculated by dividing the number of vaccinated infants by the number of same-aged infants enrolled at each site. In a previous report, CDC assessed IIS sentinel site coverage with ≥1 dose of RV among infants aged 3 months (4). In this report, however, coverage with ≥1 dose of RV was assessed among infants aged 5 months on the last day of each quarter to accommodate the June 2008 ACIP recommendation that increased the maximum age for the first dose of RV to 14 weeks and 6 days from the previously recommended maximum age of 12 weeks (1).
Using data as of June 30, 2009, CDC compared site-specific RV coverage among infants aged 5 months at each of the eight IIS sentinel sites to that of DTaP and PCV7.§ CDC also calculated unweighted average site-specific RV, DTaP, and PCV7 coverages for the eight sites; coverage for each vaccine was calculated by summing the site-specific coverages and dividing by the total number of sites (eight).
After introduction, coverage with ≥1 dose of RV among infants aged 5 months enrolled at the four continuously serving IIS sentinel sites rose quickly to about 50%--60% within the first year and then steadily (2.7% per quarter) thereafter, to 74% by the second quarter of 2009 (Figure). On June 30, 2009, a total of 23,532 infants aged 5 months were enrolled at the eight IIS sentinel sites (Table), with wide variation in number of infants per site (range: 164 to 11,767). Site-specific RV coverage ranged from 48% in Colorado to 86% in North Dakota, averaging 72% for all eight sites (Table). The site-specific coverage for ≥1 dose of DTaP or PCV7 varied by site (lowest in New York City [71% and 72%, respectively] and highest in North Dakota [93%, DTaP] and Michigan [91%, PCV7]). The average site-specific coverage for both comparison vaccines was 85%, 13 percentage points higher than the average site-specific RV coverage. The greatest difference was observed in Colorado (a 37 percentage point difference between RV and both DTaP and PCV7) and the least difference in Minnesota (a 2 percentage point difference between RV and DTaP) and in North Dakota (a 4 percentage point difference between RV and PCV7). When Colorado (an outlier) was excluded, the average site-specific coverage was 75% for RV, 85% for DTaP, and 85% for PCV7.
Reported by
DL Bartlett, MPH, Immunization Svcs Div; UD Parashar, MBBS, MM Cortese, MD, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases; DH Esposito, MD, EIS Officer, CDC.
Editorial Note
IIS sentinel sites are an important source of U.S. population--based data for the assessment of childhood and adolescent vaccination coverage. Although these data are not intended to be nationally representative, they provide quarterly vaccination information shortly after administration and are useful for identifying trends in administration of new vaccines and coverage of vaccines for which recommendations have changed over time, such as seasonal influenza vaccine (4,5). The National Immunization Survey (NIS) is nationally representative for vaccination status among children aged 19--35 months; however, NIS data on early coverage with a new vaccine given in infancy are not available until at least 2 years following introduction. NIS data for RV are expected in 2010.
Within 3 years of vaccine introduction, >70% coverage with ≥1 dose of RV among infants aged 5 months was achieved at six of the eight current IIS sentinel sites. Because IIS sentinel site systems are relatively new, data on other recently introduced vaccines administered in early infancy are not available from this source. However, based on NIS data, coverage with ≥1 dose of PCV7 by age 12 months was 88% among children born 2 years after PCV7 introduction (6), whereas coverage with ≥1 dose varicella vaccine by age 19 months was 63% among children born 3 years after introduction (7). Although coverage patterns observed following introduction of RV appear similar to the patterns for PCV7 and varicella, these comparisons should be interpreted with caution. The assessment for PCV7 was made in late infancy and was done only 2 years after introduction of the vaccine. The assessment for varicella was made in early childhood.
Despite encouraging trends, site-specific RV coverage remained on average 13 percentage points lower than that of DTaP and PCV7 in June 2009. RV is unique among vaccines recommended during infancy in having a maximum age for beginning the series (1).This age restriction could account, in part, for the lower RV coverage because an infant aged 15 weeks--5 months could still receive a first dose of DTaP or PCV7 (but not RV), according to ACIP recommendations. NIS data show that approximately 4% of infants receive their first dose of DTaP between age 15 weeks through 5 months (CDC, unpublished data; 2009). However, the difference between RV coverage and the other vaccines exceeds the difference expected based on the age restriction alone, suggesting that other barriers to receipt of RV exist. In a national survey of physicians conducted in fall 2007, 15%--19% of pediatrician and 18%--22% of family physician respondents cited perceived financial issues (e.g., reported lack of coverage by insurance companies, costs of purchasing vaccine, or lack of adequate reimbursement) and 9% of pediatrician and 25% of family physician respondents cited their own perception of vaccine safety as barriers to routinely offering RV to all eligible patients (2). A survey conducted in March 2006 to assess consumer perceptions of RV found that 29% of respondents would be unlikely to have their own child vaccinated despite 59% regarding rotavirus disease to be a very serious condition (3). An ongoing CDC-funded survey of physician attitudes and practices concerning RV is expected to update and help clarify the issues.
The findings in this report are subject to at least three limitations. First, at certain IIS sentinel sites, coverage rates for DTaP and PCV7 were lower than expected based on 2008 NIS data (8). These lower rates could result from persons who left the IIS sentinel site area before receipt of their vaccination, but who were still counted as enrolled and unvaccinated. Second, although IIS sentinel sites data are monitored for accuracy and completeness, RV might be less reliably entered into IIS than other infant vaccines because it is a relatively new vaccine. This could result in an underestimate of RV coverage levels (9). Finally, RV coverage was lowest at the small IIS sentinel site in Colorado, which represents only 3% of the total state population.
As RV coverage has increased among U.S. children, both the 2007--08 and 2008--09 rotavirus seasons were shorter in duration and diminished in magnitude compared with prevaccine seasons (10). Continued efforts to educate parents and providers about the importance of this vaccine could hasten full acceptance and help prevent severe rotavirus disease in as many U.S. children as possible. Continued monitoring of RV coverage will be crucial to provide information useful to policy makers and help focus efforts to achieve RV rates at least as high as other routinely recommended vaccines for U.S. infants.
Acknowledgments
The findings in this report are based, in part, on contributions provided by staff members at the eight IIS sentinel sites, listed at http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm.
References
- CDC. Prevention of rotavirus gastroenteritis among infants and children: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2009;58(No. RR-2).
- Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the United States. Pediatrics 2009;124:e809--16.
- Patel MM, Janssen AP, Tardif RR, Herring M, Parashar UD. A qualitative assessment of factors influencing acceptance of a new rotavirus vaccine among health care providers and consumers. BMC Pediatr 2007;7:32.
- CDC. Rotavirus vaccination coverage and adherence to the Advisory Committee on Immunization Practices (ACIP)-recommended vaccination schedule---United States, February 2006--May 2007. MMWR 2008;57:398--401.
- CDC. Influenza vaccination coverage among children and adults---United States, 2008--09 influenza season. MMWR 2009;58:1091--5.
- Nuorti JP, Martin SW, Smith PJ, Moran JS, Schwartz B. Uptake of pneumococcal conjugate vaccine among children in the 1998--2002 United States birth cohorts. Am J Prev Med 2008;34:46--53.
- CDC. 2000 National Immunization Survey child data tables: coverage levels by milestone ages; 19 months by state and IAP. Available at http://www.cdc.gov/vaccines/stats-surv/nis/tables/00/19months_iap.xls. Accessed January 14, 2010.
- CDC. 2008 National Immunization Survey child data tables: coverage levels by milestone ages; 3 months and 5 months by state and local area. Available at http://www.cdc.gov/vaccines/stats-surv/nis/data/tables_2008.htm#age. Accessed January 6, 2010.
- Khare M, Piccinino L, Barker LE, Linkins RW. Assessment of immunization registry databases as supplemental sources of data to improve ascertainment of vaccination coverage estimates in the National Immunization Survey. Arch Pediatr Adolesc Med 2006;160:838--42.
- CDC. Reduction in rotavirus after vaccine introduction---United States, 2000--2009. MMWR 2009;58:1146--9.
* IIS, also known as immunization registries, are CDC funded, locally administered, confidential, electronic data systems that collect and consolidate vaccination records on persons residing in a defined geographic region (e.g., city or state) from multiple vaccination providers and administrative sources. Additional information regarding IIS is available at http://www.cdc.gov/vaccines/programs/iis/default.htm.
† For the 2008--2012 project period, Arizona, Colorado, Michigan, Minnesota, Oregon, and Wisconsin are using subsets of their state-based IIS as their sentinel sites. North Dakota and New York City are using their entire state/city. All sites represent geographically contiguous counties, census tracts, or postal code areas. For the four states participating in the IIS Sentinel Site Project since 2006 (Arizona, Michigan, Minnesota, and Oregon), both Michigan and Oregon expanded the number of counties included, Minnesota altered which counties participated, and Arizona remained unchanged.
§ ACIP recommends that the first dose of RV, DTaP, and PCV7 each be given at age 2 months.
What is already known on this topic?
Routine vaccination against rotavirus, the most common cause of severe gastroenteritis in children, was recommended for all infants in the United States in February 2006.
What is added by this report?
Among infants aged 5 months enrolled at eight Immunization Information System sentinel sites, coverage with ≥1 dose of rotavirus vaccine has increased steadily since vaccine introduction and averaged 72% in June 2009; however, rotavirus vaccination coverage remained approximately 13 percentage points lower than that of two other routinely recommended childhood vaccinations given at the same age.
What are the implications for public health practice?
Although the trend in rotavirus vaccination coverage is encouraging, continued efforts are needed to achieve full rotavirus vaccination of U.S. infants recommended to receive rotavirus vaccine.
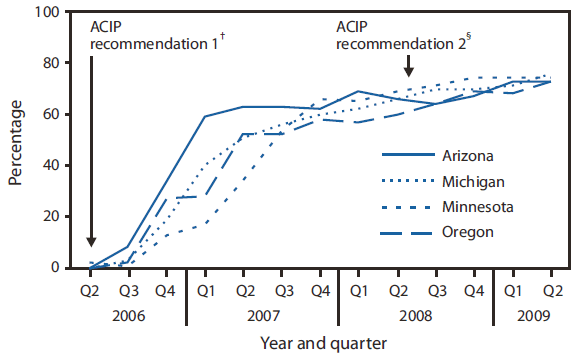
* Data reflect the four IIS sentinel sites operating continuously since the second quarter of 2006 when rotavirus vaccine was introduced. The geographic composition of some sites has changed over time.
† In February 2006, the Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination of all U.S. infants with 3 doses of RV at ages 2, 4, and 6 months.
§ In June 2008, ACIP updated its recommendations to include use of a second RV product, a 2-dose vaccine, administered at ages 2 and 4 months. The maximum age for first dose administration of RV increased from 12 weeks, as previously recommended, to 14 weeks and 6 days.
Alternate Text: The figure above shows coverage with ≥1 dose of rotavirus vaccine (RV) among infants aged 5 months, from the Immunization Information System (IIS) sentinel sites, from June 2006-June 2009. After introduction, coverage with ≥1 dose of RV among infants aged 5 months enrolled at the four continuously serving IIS sentinel sites rose quickly to about 50%-60% within the first year and then steadily (2.7% per quarter) thereafter, to 74% by the second quarter of 2009.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


