FIGURE. Percentage of invasive pneumococcal disease cases caused by serotypes covered in three different pneumococcal vaccine formulations (PCV7, PCV13, and PCV23) among adults aged ≥18 years, by age group --- Active Bacterial Core surveillance, United States, 2008
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Updated Recommendations for Prevention of Invasive Pneumococcal Disease Among Adults Using the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23)
Invasive disease from Streptococcus pneumoniae (pneumococcus) is a major cause of illness and death in the United States, with an estimated 43,500 cases and 5,000 deaths among persons of all ages in 2009 (1). This report provides updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for prevention of invasive pneumococcal disease (IPD) (i.e., bacteremia, meningitis, or infection of other normally sterile sites [2]) through use of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) among all adults aged ≥65 years and those adults aged 19--64 years with underlying medical conditions that put them at greater risk for serious pneumococcal infection. The new recommendations include the following changes from 1997 ACIP recommendations (2): 1) the indications for which PPSV23 vaccination is recommended now include smoking and asthma, and 2) routine use of PPSV23 is no longer recommended for Alaska Natives or American Indians aged <65 years unless they have medical or other indications for PPSV23. ACIP recommendations for revaccination with PPSV23 among the adult patient groups at greatest risk for IPD (i.e., persons with functional or anatomic asplenia and persons with immunocompromising conditions) remain unchanged (2). ACIP recommendations for prevention of pneumococcal disease among infants and youths aged ≤18 years using the 13-valent pneumococcal conjugate vaccine (PCV13) and PPSV23 are published separately (3).
Changes in IPD Incidence
Indirect vaccine effects (i.e., herd effects) have reduced pneumococcal infections among unvaccinated persons of all ages, including those aged ≥65 years, since introduction of the routine infant 7-valent pneumococcal conjugate vaccine (PCV7) immunization program in 2000 (4). Data from Active Bacterial Core surveillance (ABCs)* indicate that, by 2007, the overall incidence rate of IPD among persons of all ages had decreased by 45% (from 24.4. to 13.5 per 100,000 population), compared with 1998--1999 before PCV7 was introduced (4). Among persons aged 18--49 years, 50--64 years, and ≥65 years, rates of IPD decreased 40%, 18%, and 37%, respectively. The decreases resulted from reductions of 87% to 92% in cases of infection with serotypes covered in PCV7 (4). Despite the major direct and indirect PCV7 effects, IPD remains an important cause of illness and death. An estimated 43,500 cases and 5,000 deaths occurred among persons of all ages in 2009; approximately 84% of IPD cases and nearly all deaths occurred in adults (1).
Additional indirect effects can be expected to occur when the PCV13 immunization program, initiated in 2010, is fully implemented, although the magnitude of these effects is difficult to predict (3). In 2008, the serotypes covered in PCV13 caused 53%, 49%, and 44% of IPD cases among persons aged 18--49 years, 50--64 years, and ≥65 years, respectively; serotypes covered in PPSV23 caused 78%, 76%, and 66% of IPD cases among persons in these age groups (Figure).
Risk Factors for IPD Among Adults
Rates of pneumococcal infection in the United States vary among demographic groups, with higher rates among infants, young children, and older persons. The presence of certain underlying medical conditions increases the risk for pneumococcal disease and its complications (2). The risk for IPD is greatest among persons who have congenital or acquired immunodeficiency, abnormal innate immune response, human immunodeficiency virus (HIV) infection, or functional or anatomic asplenia (e.g., sickle cell disease or congenital or surgical asplenia) (Table). Alaska Native children and children among certain American Indian populations also have higher rates of IPD. Among Alaska Native and American Indian adults, the majority of IPD cases occur in persons with underlying medical conditions or other risk factors (e.g., heavy alcohol use or smoking) that are associated with increased risk for IPD in the general population (5).
From 1998--1999 to 2006--2007, the percentage of adult IPD patients with underlying medical conditions increased from 52% to 59% among those aged 18--64 years and from 69% to 81% among those aged ≥65 years. This trend suggests that adults with high-risk conditions might not have benefited as much from the indirect protective effects of childhood PCV7 immunization as persons who are relatively healthy (4).
Asthma. An estimated 7.3% of U.S. adults have active asthma.† A case-control study conducted in Tennessee, which identified cases through active, population-based and laboratory-based surveillance and verified history of asthma from the Tennessee Medicaid database, showed that among adults aged 18--49 years, IPD was more common among persons with asthma than persons without asthma (adjusted odds ratio [AOR] = 2.4; 95% confidence interval [CI] = 1.8--3.3). Among persons with high-risk asthma,§ the risk for IPD was nearly twice that for persons with low-risk asthma (6). In contrast, in a study conducted among a cohort of older veterans (average age: 53 years), persons with asthma did not have higher rates of hospitalization for pneumococcal pneumonia compared with persons in a group without asthma or chronic obstructive pulmonary disease (COPD) who were matched to the asthma patients by age, sex, and region (7). However, in the same study, hospitalization rates for pneumococcal pneumonia among persons with COPD were higher compared with persons in the control group. Because distinguishing between COPD and asthma becomes more difficult with advancing age, misclassification of persons in this study is a possibility.
Cigarette smoking. Population-based surveillance studies conducted before introduction of PCV7 consistently reported that smokers accounted for approximately half of otherwise healthy adults with IPD (8). During 2001--2003, 53% of IPD patients aged 18--64 years were current cigarette smokers (CDC, ABCs unpublished data). In a multicenter, population-based, case-control study in which IPD patients were identified through ABCs, the risk for IPD among immunocompetent cigarette smokers aged 18--64 years was four times the risk for controls who had never smoked (AOR = 4.1; CI = 2.4--7.3) (9). Significant dose-response relationships with risk for IPD also were observed for number of cigarettes smoked and pack-years of smoking (9). Subsequent studies confirmed that smoking also increases the risk for IPD among other groups, including immunocompromised persons (10).
PPSV23 Efficacy and Effectiveness
Evaluations of PPSV23 efficacy and effectiveness among persons in recommended target groups have yielded contradictory conclusions for prevention of nonbacteremic pneumococcal pneumonia; however, most study results are consistent with protection against IPD among generally healthy young adults and among the general population of older persons. Observational studies have suggested effectiveness estimates ranging from approximately 50% to 80% for prevention of IPD among immunocompetent older adults and adults with various underlying illnesses, supporting the recommendations for using PPSV23 to prevent IPD (11). However, effectiveness has not been demonstrated among immunocompromised persons or very old persons. A recent meta-analysis of 15 randomized controlled trials (RCTs) and seven nonrandomized observational studies of PPSV23 efficacy and effectiveness suggested an overall efficacy of 74% against IPD (CI = 56%--85%), based on pooled results of 10 of the RCTs (12). Analysis of the results from the seven observational studies yielded a pooled vaccine effectiveness estimate of 52% (CI = 39%--63%). In contrast, a recent meta-analysis that included six RCTs estimated the combined PPSV23 efficacy against pneumococcal bacteremia at only 10%, with a very wide CI (CI = -77%--54%) (13). The large difference in findings from these two meta-analyses might be related to inclusion of different trials.
Recommendations for Use of PPSV23
At its June and October 2008 meetings, ACIP approved new and revised recommendations for the use of PPSV23 to prevent IPD among adults aged <65 years. ACIP concluded that asthma is an independent risk factor for IPD and should be included in the group of chronic pulmonary diseases (e.g., COPD and emphysema) that are indications for PPSV23 (Table); thus, ACIP recommended that persons aged 19--64 years who have asthma should receive a single dose of PPSV23 (Box). ACIP also concluded that adults who smoke cigarettes are at significantly increased risk for IPD and recommended that persons aged 19--64 years who smoke cigarettes should receive a single dose of PPSV23 and smoking cessation guidance (Box).
ACIP also revised its recommendation for use of PPSV23 among American Indians and Alaska Natives. Routine use of PPSV23 is no longer recommended for persons aged <65 years in these populations unless they have a medical condition or other indication for PPSV23. However, in certain situations, public health authorities may recommend PPSV23 for Alaska Natives and American Indians aged 50--64 years who are living in areas where the risk for IPD is increased.
All persons should be vaccinated with PPSV23 at age 65 years. Those who received PPSV23 before age 65 years for any indication should receive another dose of the vaccine at age 65 years or later if at least 5 years have passed since their previous dose. Those who receive PPSV23 at or after age 65 years should receive only a single dose.
Revaccination. ACIP recommendations for revaccination remain unchanged from the 1997 recommendations (2). For most persons for whom PPSV23 is indicated, ACIP does not recommend routine revaccination. A second dose of PPSV23 is recommended 5 years after the first dose for persons aged 19--64 years with functional or anatomic asplenia and for persons with immunocompromising conditions (Table). ACIP does not recommend multiple revaccinations because of insufficient data regarding clinical benefit, particularly the degree and duration of protection, and safety.
Smoking cessation. Quitting smoking reduces the risk for pneumococcal disease. One study found that the risk for IPD was reduced by approximately 14% each year after quitting smoking and returned to a risk similar to that for persons who had never smoked in approximately 13 years (9). ACIP emphasizes that smoking cessation guidance should be part of the therapeutic plan for smokers regardless of immunization status. Professional organizations such as the Infectious Disease Society of America and American Thoracic Society also recommend smoking cessation counseling and pneumococcal vaccination for smokers who are hospitalized with community-acquired pneumonia (14). Clinical practice guidelines from the U.S. Public Health Service for treating tobacco use and dependence are available at http://surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf.
Reported by
JP Nuorti, MD, DSc, CG Whitney, MD, Div of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC, for the ACIP Pneumococcal Vaccines Working Group.
References
- CDC. Active Bacterial Core Surveillance (ABCs) Report: Emerging Infections Program Network. Streptococcus pneumoniae, provisional-2009. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Available at http://www.cdc.gov/abcs/reports-findings/survreports/spneu09.pdf.
- CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-8).
- CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children---Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59:258--61.
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201:32--41.
- Singleton RJ, Butler JC, Bulkow LR, et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska Native adults. Vaccine 2007;25:2288--95.
- Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005;352:2082--90.
- Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007;22:62--7.
- Plouffe JF, Breiman RF, Facklam RR. Bacteremia with Streptococcus pneumoniae: implications for therapy and prevention. Franklin County Pneumonia Study Group. JAMA 1996;275:194--8.
- Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681--9.
- Breiman RF, Keller DW, Phelan MA, et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med 2000;160:2633--8.
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008;83:373--84.
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008;(1):CD000422.
- Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009;180:48--58.
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(Suppl 2):S27--72.
* Additional information available at http://www.cdc.gov/abcs/index.html.
† Additional information available at http://www.cdc.gov/asthma/nhis/07/data.htm.
§ Defined as persons with asthma that required at least one of the following: 1) admission for asthma to a hospital or visit to an emergency department; 2) receipt of a prescription for a course of corticosteroids as rescue therapy or a long-term course (120 days or more) of oral corticosteroids; or 3) three or more prescriptions for β-agonists in the year preceding enrollment in the study.
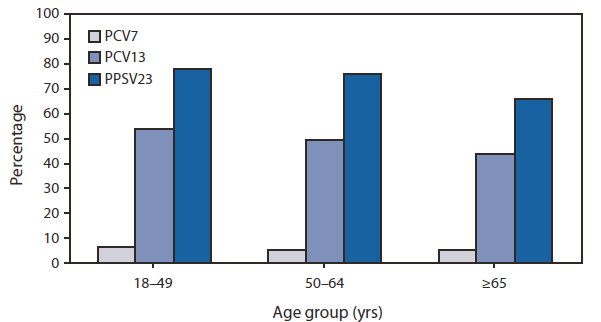
Abbreviations: PCV7 = 7-valent pneumococcal conjugate vaccine. PCV13 = 13-valent pneumococcal conjugate vaccine. PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
Alternate Text: The figure above shows the percentage of invasive pneumococcal disease cases caused by serotypes covered in three different pneumococcal vaccine formulations (PCV7, PCV13, and PCV23) among adults aged ≥18 years, by age group in the United States in 2008. Results were derived from Active Bacterial Core surveillance.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


