Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Nonpolio Enterovirus and Human Parechovirus Surveillance --- United States, 2006--2008
Enteroviruses, members of the Picornaviridae family, are common viruses associated with clinical manifestations ranging from mild respiratory symptoms to serious conditions, including aseptic meningitis, encephalitis, neonatal sepsis, and acute flaccid paralysis. Approximately 100 serotypes of nonpolio enteroviruses have been recognized (1), and some viruses previously classified as enteroviruses, namely echovirus 22 and 23, recently have been reclassified as human parechoviruses (HPeVs), a different genus within the Picornaviridae family. This report describes trends in nonpolio enterovirus and HPeV detections during 2006--2008, based on data from two laboratory-based surveillance systems, the National Enterovirus Surveillance System (NESS) and, for the first time, the National Respiratory and Enteric Virus Surveillance System (NREVSS). As in previous years, approximately 70% of detections occurred during July--October, the peak enterovirus season. The five most common enterovirus serotypes (coxsackievirus B1 [CVB1], echovirus 6, echovirus 9, echovirus 18, and coxsackievirus A9) accounted for 54% of total serotyped detections. During 2006--2008, southern states reported the most serotyped enterovirus detections, followed by midwestern states, western states, and the northeastern states. In 2007 and 2008, CVB1 was the predominant serotype detected, accounting for 24% and 19% of overall detections, respectively. In 2007, CVB1 was implicated in an outbreak of serious neonatal infections in the United States (2). Understanding trends in enterovirus and HPeV circulation can help clinicians decide when to test for these infections. Also, more timely reporting of data could help public health officials recognize outbreaks associated with these viruses.
NESS, initiated in 1961, is a passive, voluntary surveillance system that monitors laboratory detections of enteroviruses in the United States. Participating laboratories are encouraged to report enterovirus detections by serotype, specimen type, collection date, age of patient, and sex of patient to CDC monthly. Enterovirus serotyping is performed by sequencing the genome region encoding the VP1 capsid protein by immunofluorescence using type-specific monoclonal antibodies, or by neutralization with type-specific polyclonal antisera.
NREVSS is a passive, voluntary, laboratory-based surveillance system that tracks temporal and geographic trends in the circulation of respiratory and enteric pathogens. NREVSS began collecting enterovirus reports in July 2007. It collects the number of enterovirus tests and the proportion that are positive, by specimen site and collection date; no serotyping, demographic data, or clinical data are reported. All participating laboratories that reported at least one enterovirus-positive specimen were included in this analysis. Enteroviruses were detected by cell culture or nucleic acid detection (polymerase chain reaction).
During 2006--2008, enterovirus and HPeV detections were reported from 49 states through one or both of these surveillance systems during the years specified (Figure 1). A total of 20 laboratories (including 18 public health laboratories, one private reference laboratory, and CDC's Picornavirus Laboratory) reported results to NESS. Public health and private laboratories without the capacity to serotype send specimens to CDC Picornavirus Laboratory for serotyping. A total of 1,632 enterovirus or HPeV detections were reported to NESS during this period (920 from public health laboratories, 661 from the one reference laboratory, and 51 from CDC's Picornavirus Laboratory). Of these detections, 1,103 (68%) were reported during July--October. The age of patients for whom age was known (1,415 [87%]) ranged from <1 month to 79 years, with a mean age of 9 years and a median age of 2 years. Children aged ≤1 year accounted for 660 (47%) of these 1,415 enterovirus or HPeV detections for which the age of patient was known. Cerebral spinal fluid was the most common source for detections, accounting for 743 (51%) of the 1,468 reports of known specimen type, followed by 324 (22%) detections from throat-nasopharyngeal specimens, 268 (17%) from stool-rectal swabs, and 133 (10%) from tissue specimens.
Enterovirus or HPeV serotypes were specified for 1,171 (72%) NESS reports. By region,* southern states had the most serotyped detections reported, accounting for 418 of 1,167 (36%) reports for which state information was provided, followed by 373 (32%) detections from midwestern states, 222 (19%) from western states, and 154 (13%) from northeastern states. The five most common enterovirus serotypes accounted for 54% of total detections with a known serotype in 2006--2008 (Table). Overall, during 2006--2008, CVB1 was the most commonly detected enterovirus identified in 235 (17%) of 1,171 specimens tested (Table). In 2007, CVB1 was detected in 22 mostly southern and western states; two states reported 70 (51%) of 137 detections. In 2008, CVB1 was detected in 10 states; one state reported 15 (34%) of 44 detections. During 2006--2008, three public health laboratories and CDC's Picornavirus Laboratory reported a total of 21 HPeV type 1 (HPeV1) detections. HPeV1 was one of the 15 most common enteroviruses reported during the surveillance period but was detected in <2% of specimens (Table). During the surveillance period, only 14 cases of enterovirus 71 (EV71), a virus that has caused widespread outbreaks of hand, foot, and mouth disease in several Asian countries (3), were reported to NESS, and it was not included as one of the 15 most common serotypes reported.
During July 2007--December 2008, a total of 3,192 (3%) of the 108,798 reports sent to NREVSS were positive for enterovirus. One hundred laboratories reported testing for enteroviruses to NREVSS. During this period, the highest proportion of detections was reported during July--October (Figure 2). The proportion of enterovirus-positive specimens was similar across regions.
Reported by
GR Villarruel, MPH, GE Langley, MD, MS Oberste, PhD, M Pallansch, PhD, Div of Viral Diseases, National Center for Immunizations and Respiratory Diseases, CDC.
Editorial Note
The findings in this report are consistent with previous trends regarding the most commonly detected enterovirus serotypes (4). During 2006--2008, CVB1 became the predominant enterovirus serotype identified, found in several states, and concentrated in a few. Common clinical presentations of CVB1 include aseptic meningitis, myocarditis, pleurodynia, and hand, foot, and mouth disease. CVB1 generally shows an epidemic pattern of circulation with irregular intervals of increased circulation usually lasting 2--3 years (4). During 1970--2005, CVB1 accounted for approximately 2% of reports with known serotype, with an increase of CVB1 observed in the early 1990s and then again in the early 2000s (4). In 2007, increased detections of CVB1 reported to NESS led to an investigation that identified severe neonatal disease and deaths associated with CVB1 infection in multiple states (2,5).
Since 1997, EV71 has caused widespread outbreaks of hand, foot, and mouth disease in several Asian countries (3). A small proportion of cases have resulted in encephalitis and death. In the United States, small clusters of serious disease were detected during 2003--2005 (6). Although there was an increase in reported EV71 detections in the United States during 2006--2008, EV71 detections were uncommon.
HPeV1 usually has been associated with mild gastrointestinal and respiratory symptoms, meningitis, and neonatal sepsis (7). HPeV1 was one of the 15 most common detections during 2006--2008; no other HPeV types were reported during that period. HPeV cannot be detected by EV-specific assays. CDC's Picornavirus Laboratory has performed the majority of HPeV typing reported to NESS but has worked with clinical and state laboratories to enhance their HPeV diagnostic and molecular typing assays (8) to improve detections and enhance parechovirus surveillance.
The findings in this report are subject to at least four limitations. First, enteroviral infections other than poliovirus infections are not nationally notifiable in the United States. NESS is a passive system that relies on voluntary participation from laboratories, so findings are not necessarily representative of national or regional enterovirus activity. Although there might be more reports emanating from one state or region, it might not represent an increased burden of disease in that state or region. Second, the findings are limited by the lack of clinical information; however, most detections likely represent serious disease because cerebral spinal fluid was the most common source of detection. Third, most testing is performed during the summer months; circulation during other parts of the year might go undetected. Finally, although monthly NESS reporting is encouraged, not all participating laboratories submit timely data, which can delay accurate reporting.
NESS could be improved with more regular reporting by current laboratories and by increasing the number of participating laboratories. NREVSS provides enterovirus activity over a wider geographic area because more laboratories participate in the system, but it does not provide serotype or demographic information. The combined systems provide the best available data on enterovirus circulation in the United States.
Since July 2009, a simplified, Internet-based NESS system has allowed participating laboratories to easily input enterovirus detection data and to analyze national and state-based trends in enterovirus surveillance, by serotype. Additional information about this system is available by e-mail (ness@cdc.gov).
Acknowledgments
The findings in this report are based, in part, on contributions by staff members of participating NREVSS laboratories and state virology laboratories reporting to NESS.
References
- Pallansch MA, Roos RP. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Knipe DM, Howley PM, Griffin DE, et al, eds. Fields Virology, 4th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2001:723--75.
- CDC. Increased detections and severe neonatal disease associated with coxsackievirus B1 infection---United States, 2007. MMWR 2008;57:553--6.
- CDC. Deaths among children during an outbreak of hand, foot, and mouth disease---Taiwan, Republic of China, April--July 1998. MMWR 1998;47:629--32.
- CDC. Enterovirus surveillance---United States, 1970--2005. MMWR 2006;55(No. SS-08).
- Wikswo ME, Khetsuriani N, Fowlkes AL, et al. Increased activity of coxsackievirus B1 strains associated with severe disease among young infants in the United States, 2007--2008. Clin Infect Dis 2009;49:e44--51.
- Perez-Velez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis 2007;45:9507.
- Harvala H, Wolthers KC, Simmonds P. Parechoviruses in children: understanding a new infection. Curr Opin Infect Dis 2010;23:224--30.
- Nix WA, Maher K, Johansson ES, et al. Detection of all known parechoviruses by real-time PCR. J Clin Microbiol 2008;46:2519--24.
* Midwest: Illinois, Indiana, Kansas, Michigan, Minnesota, Missouri, Ohio, and Wisconsin; Northeast: Massachusetts, New Jersey, and New York; South: Florida, Georgia, Kentucky, Louisiana, Maryland, North Carolina, Oklahoma, and Tennessee; West: Alaska, Arizona, California, Colorado, Hawaii, Nevada, New Mexico, Oregon, Utah, and Washington.
What is already known on this topic?
Approximately 100 serotypes of nonpolio enteroviruses have been recognized and are associated with mild to serious conditions, including aseptic meningitis, encephalitis, neonatal sepsis, and acute flaccid paralysis, especially during the summer and fall months.
What is added by this report?
During 2006--2008, the five most frequently detected enteroviruses were coxsackievirus B1 (CVB1), echovirus 6, echovirus 9, echovirus 18, and coxsackievirus A9; these accounted for 54% of total known serotyped detections during that period. In 2007 and 2008, CVB1 became the predominant serotype detected, accounting for 24% and 19% of overall detections, respectively.
What are the implications for public health practice?
Understanding trends in enterovirus and human parechovirus circulation can help clinicians decide when to test for these infections and can guide public health officials to recognize outbreaks associated with these viruses, as was the case with an outbreak of serious neonatal infections associated with CVB1 in 2007.
FIGURE 1. Method of reporting enterovirus detections, by state --- National Enterovirus Surveillance System (NESS) and National Respiratory and Enteric Virus Surveillance System (NREVSS), United States, 2006--2008
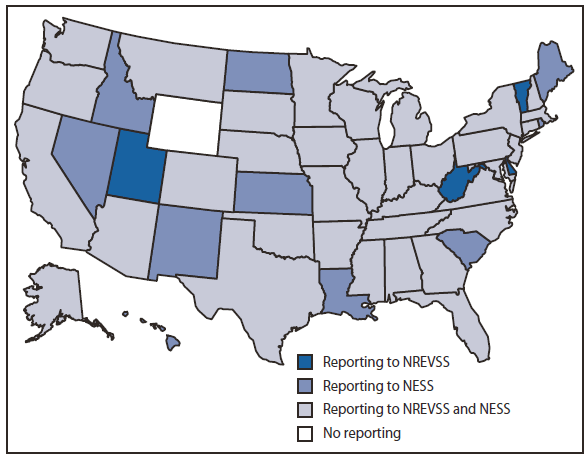
Alternate Text: The figure above shows the method of reporting enterovirus detections, by state, in the United States during 2006-2008. Enterovirus and HPeV detections were reported from 49 states through one or both of these surveillance systems during the years specified.
FIGURE 2. Percentage of specimens testing positive for enterovirus, by month of report --- National Respiratory and Enteric Virus Surveillance System (NREVSS), United States, July 2007--December 2008
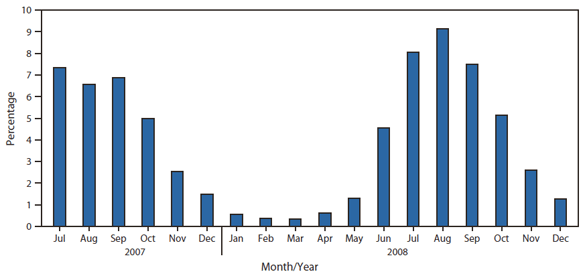
Alternate Text: The figure above shows the percentage of specimens testing positive for enterovirus, by month of report, in the United States during July 2007-December 2008. A total of 3,192 (3%) of the 108,798 reports sent to NREVSS were positive for enterovirus. One hundred laboratories reported testing for enteroviruses to NREVSS. During this period, the highest proportion of detections was reported during July-October.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


