FIGURE. Pathologic findings indicating that a deceased researcher had septicemic plague, not pneumonic plague* --- Chicago, Illinois, 2009
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Fatal Laboratory-Acquired Infection with an Attenuated Yersinia pestis Strain --- Chicago, Illinois, 2009
On September 18, 2009, the Chicago Department of Public Health (CDPH) was notified by a local hospital of a suspected case of fatal laboratory-acquired infection with Yersinia pestis, the causative agent of plague. The patient, a researcher in a university laboratory, had been working along with other members of the laboratory group with a pigmentation-negative (pgm-) attenuated Y. pestis strain (KIM D27). The strain had not been known to have caused laboratory-acquired infections or human fatalities. Other researchers in a separate university laboratory facility in the same building had contact with a virulent Y. pestis strain (CO92) that is considered a select biologic agent; however, the pgm- attenuated KIM D27 is excluded from the National Select Agent Registry (1). The university, CDPH, the Illinois Department of Public Health (IDPH), and CDC conducted an investigation to ascertain the cause of death. This report summarizes the results of that investigation, which determined that the cause of death likely was an unrecognized occupational exposure (route unknown) to Y. pestis, leading to septic shock. Y. pestis was isolated from premortem blood cultures. Polymerase chain reaction (PCR) identified the clinical isolate as a pgm- strain of Y. pestis. Postmortem examination revealed no evidence of pneumonic plague. A postmortem diagnosis of hereditary hemochromatosis was made on the basis of histopathologic, laboratory, and genetic testing. One possible explanation for the unexpected fatal outcome in this patient is that hemochromatosis-induced iron overload might have provided the infecting KIM D27 strain, which is attenuated as a result of defects in its ability to acquire iron, with sufficient iron to overcome its iron-acquisition defects and become virulent (2). Researchers should adhere to recommended biosafety practices when handling any live bacterial cultures, even attenuated strains, and institutional biosafety committees should implement and maintain effective surveillance systems to detect and monitor unexpected acute illness in laboratory workers.
Case Report
On September 10, 2009, the researcher, a man aged 60 years with insulin-dependent diabetes mellitus, was evaluated at an outpatient clinic for fever, body aches, and cough of approximately 3 days duration. A clinic physician suspected influenza or other acute respiratory infection and referred the patient to an emergency department (ED) for further evaluation; however, the patient did not seek further care at that time. On September 13, the patient was brought by ambulance to a Chicago hospital ED because of fever, cough, and worsening shortness of breath. Paramedics recorded an oxygen saturation level of 92%, and oxygen was administered via mask.
Upon arrival at the ED, the patient was noted to be alert and able to converse, with a temperature of 100.9oF (38.3oC), pulse of 106 beats per minute, respiratory rate of 42 breaths per minute, and blood pressure of 106/75 mm/Hg. Examination revealed distant breath sounds, abdominal distention, peripheral cyanosis, and trace pedal edema; no lymphadenopathy, rash, or jaundice was noted. A chest radiograph revealed normal lung fields; however, the patient continued to have labored breathing and required supplemental oxygen. Blood chemistries showed renal failure (creatinine: 6.5 mg/dL; blood urea nitrogen: 73 mg/dL), incipient acidosis (bicarbonate: 17 mEq/L; PaCO2: 31mmHg; pH: 7.36), and elevated liver function enzymes (aspartate aminotransaminase [AST]: 794 IU/L; alanine aminotransaminase [ALT]: 160 IU/L). Complete blood count showed severe leukocytosis (white blood cells: 79.2 103/mL) with a left shift (22% band forms) and hemoglobin level and platelet count within normal limits. Extracellular bacteria were noted on a peripheral blood smear.
The patient initially was treated with diuretics for suspected congestive heart failure and later with intravenous antibiotics (vancomycin and piperacillin/tazobactam) once infection was suspected. At approximately 12 hours after presentation, the patient had worsening respiratory distress and was intubated. He died 1 hour later of cardiac arrest, despite resuscitation attempts.
The patient had last worked in the laboratory on September 4. On September 10, he notified his supervisor about his illness to explain his absence from work. Whether the patient himself suspected his symptoms were consistent with plague is not known; however, existing laboratory policy called for laboratory workers with illness consistent with plague to report to the university's occupational health clinic (or to the ED). The patient's occupation was not documented in the records of either the outpatient clinic he visited or the hospital ED.
On September 14, blood cultures drawn the previous day yielded gram-negative bacilli (four of four bottles), gram-positive cocci (three of four bottles), and yeast (one of four bottles and presumed to be a contaminant). On September 15, the clinical laboratory identified the gram-positive cocci as nutritionally variant streptococci (NVS). An autopsy performed the same day identified no signs of pneumonia, bowel perforation, or endocarditis, which is often associated with NVS infection. Efforts to identify the slow-growing, gram-negative organism were under way when, on September 16, an ED physician learned that the patient had worked in a laboratory that conducted research on select biologic agents and notified the hospital clinical laboratory. On the morning of September 18, 16S ribosomal DNA sequencing performed by the hospital clinical laboratory narrowed the identity of the gram-negative bacilli to either Y. pestis or Y. pseudotuberculosis. That same day, hospital infection control staff members notified CDPH of the suspected Y. pestis case.
Epidemiologic and Environmental Investigation
On September 18, CDPH, IDPH, CDC, and the university initiated a joint investigation to determine the source of Y. pestis infection, identify any potential additional infections, and implement prevention and control measures. Because inhalation exposure could not be excluded, antimicrobial prophylaxis was offered to all staff members at the research laboratory in which the patient worked and to his close contacts. A close contact was defined as a person who had been within 6 feet of the patient or who had handled his blood or tissue samples during September 7--18, 2009. Unless contraindications existed, a 7-day course of doxycycline was prescribed for prophylaxis. All 30 coworkers were prescribed prophylaxis during September 19--20. One household contact and 64 other close contacts, including medical, laboratory, and pathology personnel, were identified, and 61 (94%) accepted prophylaxis during September 18--20. No additional Y. pestis infections have been identified.
An assessment of the laboratory environment identified no major deficiencies in laboratory engineering controls. A review of Occupational Safety and Health Administration Form 300 logs identified no recent work-related injuries or illnesses among workers in this laboratory. A review of attendance records for university biosafety training identified deficiencies in staff attendance (including the patient) at a number of required biosafety courses. The patient's family members and coworkers were asked about knowledge of any possible exposure events, such as a needle puncture or splash of liquid to the face, and none were reported. Interviews with laboratory coworkers revealed that the patient inconsistently complied with the laboratory policy to wear gloves while handling Y. pestis KIM D27 bacterial cultures.
Laboratory and Pathologic Testing
Preliminary PCR and microbiologic analyses conducted by the university research laboratory identified the organism isolated from the patient as a pgm- attenuated strain of Y. pestis (3), consistent with the KIM D27 strain with which the patient worked, and not the virulent Y. pestis strain CO92, which was used in a separate laboratory facility on another floor. The IDPH Division of Laboratories confirmed the infecting organism as Y. pestis. CDC independently isolated Y. pestis from an aliquot of one of the original blood culture bottles and confirmed the absence of the pgm locus. CDC also identified a chloramphenicol resistance gene (a common, laboratory-based resistance marker) that was not in the original laboratory stock strain, suggesting that the infecting strain had been modified as part of routine laboratory research.
To determine whether new virulence mechanisms had been acquired or genetically inserted into the infecting strain, CDC compared the virulence of the infecting strain with that of CO92 from CDC culture archives and the original, attenuated KIM D27 stock strain from the patient's laboratory. Male Swiss Webster mice were inoculated subcutaneously with varying doses of bacteria ranging from 103 to 108 colony-forming units (CFUs) for the infecting strain and the parental KIM D27 strain, and from 102 to 103 for CO92. Virulence was measured by determining the median lethal dose (LD50). The LD50 of CO92 was ≤100 CFUs. In contrast, an LD50 was not achieved for either the infecting strain or KIM D27; <3% of the mice died after injection with doses as high as 108 CFUs. Such findings suggest that the strain with which the patient was infected was attenuated and that no new virulence mechanisms were acquired by or engineered into the infecting strain.
Histopathologic examination of the patient's lung tissue at CDC revealed preserved alveolar structure without inflammatory infiltrates that would have indicated pneumonic processes consistent with plague (Figure). Immunohistochemical tests using an anti--Y. pestis mouse monoclonal antibody revealed abundant staining of Y. pestis in blood vessels of multiple organs, consistent with septicemic plague. Histologic staining revealed abnormal iron deposits in the liver, but not in pancreatic or cardiac tissues. Postmortem testing of blood samples revealed a total serum iron of 541 mcg/dL (reference range: 40--160 mcg/dL), iron saturation of 83.5% (reference range: 14%--50%), and total iron binding capacity of 648 mcg/dL (reference range: 230--430 mcg/dL). Genetic testing revealed that the patient was homozygous for the C282Y mutation of the HFE gene, confirming a postmortem diagnosis of hereditary hemochromatosis (4). Investigators found no evidence that the researcher knew he had hemochromatosis or that he exhibited any symptoms of this condition.
Reported by
K Ritger, MD, S Black, MD, K Weaver, MPH, J Jones, MD, Chicago Dept of Public Health; S Gerber, MD, Cook County Dept of Public Health; C Conover, MD, K Soyemi, MD, Illinois Dept of Public Health. K Metzger, MPH, CDC/CSTE Applied Epidemiology Fellow; B King, MPH, National Institute for Occupational Safety and Health; P Mead, MD, C Molins, PhD, M Schriefer, PhD, Div of Vector-Borne Diseases, W-J Shieh, MD, S Zaki, MD, Div of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases; A Medina-Marino, PhD, EIS Officer, CDC.
Editorial Note
This report describes the first known fatality from a laboratory-acquired infection with attenuated Y. pestis. The last known laboratory-acquired infection with Y. pestis in the United States occurred in 1959 and was caused by inhalation of a virulent strain (5). Pathology findings for the case described in this report are consistent with septicemic plague and inconsistent with pneumonic plague, suggesting a transdermal or mucosal route of infection. However, a recent study (6) demonstrated that intranasal infection of mice with KIM D27 can result in persistent colonization of the lung vasculature, subsequent dissemination of infection, and eventual death without classic pathologic signs of pneumonic plague. Consequently, a respiratory route of infection cannot be ruled out definitively. Although the route of transmission for the infection remains unclear, deficiencies in biosafety practices, including inconsistent use of gloves, could have resulted in inadvertent transdermal exposure. The severe outcome experienced by the patient was unexpected, given that he had worked with an attenuated Y. pestis strain that 1) is widely used by laboratory researchers, 2) has not been associated with previous laboratory-acquired infections or fatalities, and 3) is excluded from select biologic agent requirements (1). Although NVS bacteremia cannot be excluded as a contributing factor in the patient's death, the clinical course, lack of endocarditis, and the visualization of Y. pestis in blood vessels throughout the body strongly support gram-negative sepsis as the cause of death.
Hemochromatosis, an iron-overload disease (7), is characterized by increased iron absorption and progressive iron storage in multiple organs. Persons with hemochromatosis are especially susceptible to infection with Vibrio vulnificus, Listeria monocytogenes, Yersinia enterocolitica, and Yersinia pseudotuberculosis (7). Animal studies have shown that the virulence of pgm- Y. pestis strains can be enhanced by the simultaneous injection of iron into experimental animals (2). Conceivably, hemochromatosis-induced iron overload might have a similar effect, enhancing the virulence of the infecting KIM D27 strain by compensating for its iron-acquisition defects (2). Although iron deposition in pancreatic tissue is known to cause bronze diabetes, pathologic examination ruled out iron deposition as the cause of this patient's diabetes. Nonetheless, diabetes is a known risk factor for increased severity and complications from bacterial infections (8).
Research using animal models of hemochromatosis (9) is under way to better understand susceptibility to infection with bacteria attenuated as a result of defects in iron acquisition. Researchers working with attenuated Y. pestis and other potentially infectious material should always use at least biosafety level 2 practices (10), and laboratory managers should ensure that staff adheres to recommended biosafety practices. Institutional safety committees should implement and maintain effective surveillance programs to identify and monitor acute illness among laboratory workers, and health-care providers should routinely inquire about occupational exposures when evaluating patients.
References
- US Department of Health and Human Services, US Department of Agriculture. National Select Agent Registry: select agents exclusions. Washington, DC: US Department of Health and Human Services, US Department of Agriculture; 2010. Available at http://www.selectagents.gov/select%20agents%20and%20toxins%20exclusions.html. Accessed February 18, 2011.
- Jackson S, Burrows TW. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol 1956;37:577--83.
- Perry RD, Fetherston JD. Yersinia pestis---etiologic agent of plague. Clin Microbiol Rev 1997;10:35--66.
- Andrews NC. Disorders of iron metabolism. N Engl J Med 1999;341:1986--95.
- Burmeister RW, Tigertt WD, Overholt EL. Laboratory-acquired pneumonic plague: report of a case and review of previous cases. Ann Intern Med 1962;56:789--800.
- Lee-Lewis H, Anderson DM. Absence of inflammation and pneumonia during infection with nonpigmented Yersinia pestis reveals a new role for the pgm locus in pathogenesis. Infect Immun 2010;78:220--30.
- Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis 2007;11:482--7.
- Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999;341:1906--12.
- Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest 2005;115:2187--91.
- US Department of Health and Human Services, CDC, National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 5th ed. Atlanta, GA: US Department of Health and Human Services, CDC, National Institutes of Health; 2009. Available at http://www.cdc.gov/biosafety/publications/bmbl5. Accessed February 18, 2011.
What is already known on this topic?
The last known laboratory-acquired infection with Yersinia pestis in the United States occurred in 1959.
What is added by this report?
This case report describes the first reported fatality from a laboratory-acquired infection with an attenuated strain of Y. pestis.
What are the implications for public health practice?
Under certain environmental and host conditions, infection with attenuated bacteria might result in severe disease. Researchers always should adhere to recommended use of personal protective equipment. Unexpected acute illness in a laboratory worker should be reported to the institution and health-care providers so that the differential diagnosis can be expanded to include diseases occurring as a result of occupational exposures.
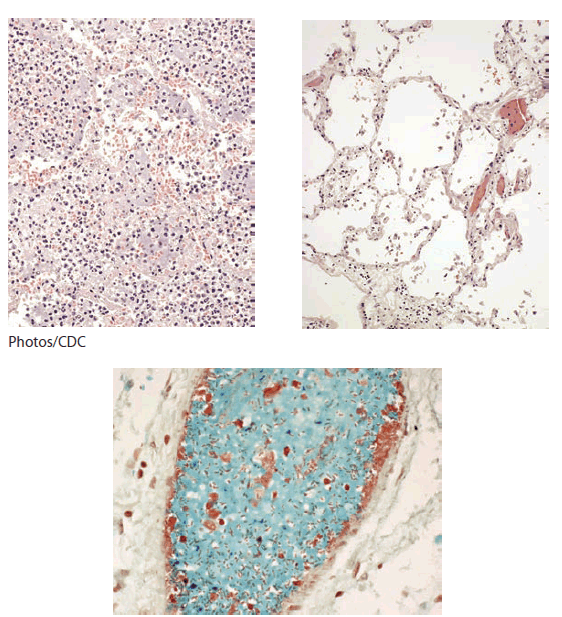
* Investigators compared lung pathology images of a patient with primary pneumonic plague (top, left), with that of the deceased researcher (top, right). Lung tissue of the patient with primary pneumonic plague shows extensive inflammation and no open air space. Postmortem lung tissue from the deceased researcher shows open air spaces and preserved alveolar structure, indicating the deceased did not have pneumonic processes consistent with plague. Magnification of postmortem lung tissue (bottom) revealed gram-negative bacteria (red, rod-shaped) in the lumen of a pulmonary blood vessel. Subsequent immunostaining with a Yersinia pestis antibody confirmed Y. pestis only in blood vessels of numerous tissues, consistent with septicemic plague.
Alternate Text: The figure above shows pathologic findings indicating that the deceased researcher had septicemic plague, not pneumonic plague, in the fatal case of laboratory-acquired infection with an attenuated Yersinia pestis strain in Chicago in 2009.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


