Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Outbreak of Invasive Listeriosis Associated with the Consumption of Hog Head Cheese --- Louisiana, 2010
During January--June 2010, a total of 14 cases of laboratory-confirmed invasive listeriosis were reported to the Louisiana Office of Public Health (OPH). Isolates of Listeria monocytogenes from the blood samples of eight patients were identified as serotype 1/2a and had pulsed-field gel electrophoresis (PFGE) pattern combinations that were indistinguishable from one another. The detection of this cluster prompted an investigation in coordination with CDC, the Louisiana Department of Agriculture and Forestry (LDAF), and the U.S. Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS). In-depth epidemiologic and environmental investigations of the cluster were initiated on July 26, including food history interviews of four patients. Three patients reported eating hog head cheese (a meat jelly made from swine heads and feet); the product was purchased at two grocery stores in Louisiana. A traceback investigation determined that a single brand of hog head cheese was common between the two grocery stores. L. monocytogenes serotype 1/2a was cultured from one of three product samples and from two of 16 environmental samples collected by LDAF at the processing establishment; the product and one of the two environmental samples yielded isolates with PFGE pattern combinations that were indistinguishable from the patient isolates. On August 14, LDAF coordinated a voluntary recall of approximately 500,000 pounds of hog head cheese and sausage because of possible contamination with L. monocytogenes. This is the first published report of an invasive listeriosis outbreak associated with hog head cheese, which is a ready-to-eat (RTE) meat. USDA-FSIS has a "zero tolerance" policy for L. monocytogenes contamination of RTE food products (1), requesting recall of such products at any detectable level of L. monocytogenes contamination. LDAF imposes and enforces equivalent requirements in state-inspected establishments.
Invasive listeriosis has been nationally notifiable since 1999. In 2003, the Council of State and Territorial Epidemiologists recommended prompt, routine interviews of all patients using a standardized questionnaire and forwarding all L. monocytogenes isolates from clinical laboratories for PFGE subtyping at public health laboratories (2). Accordingly, the Louisiana OPH collects demographic and clinical information for all reported cases of invasive listeriosis. Patients are interviewed immediately for food histories using CDC's Listeria Initiative questionnaire.* Patient isolates are sent to the Public Health Central Laboratory at OPH for confirmation and PFGE characterization.
Louisiana OPH epidemiologists noted that 14 cases of invasive listeriosis had been reported during January--June 2010, which exceeded the state's average of five cases reported during each January--June period during the previous 3 years. For this investigation, a cluster-associated case was defined as isolation of L. monocytogenes serotype 1/2a from a normally sterile site (e.g., blood or cerebrospinal fluid) or from placental or fetal tissue (in the setting of miscarriage or stillbirth) since January 1, 2010, and PFGE pattern combination GX6A16.0001 and GX6A12.0001.
Eight patients had illnesses that met the case definition. Their median age was 64 years (range: 38--93 years). Six patients were men; no patients were pregnant. Six patients had one or more underlying medical conditions (i.e., human immunodeficiency virus [HIV] infection, alcohol abuse, cancer, and diabetes mellitus). Illness onsets occurred from February 18 to June 16 (Figure). Signs and symptoms included fever (n = 6 patients), altered mental status (n = 3), diarrhea (n = 3), vomiting (n = 3), and weakness (n = 2). Seven patients were hospitalized; two patients died.
OPH epidemiologists obtained food histories from four patients; the remaining patients could not be reached for interview because of their illness or death. Two patients initially reported eating hog head cheese purchased from the same grocery store. Upon re-interview, a third patient also reported eating hog head cheese purchased from a grocery store in another city. A fourth patient could not be reached for re-interview but had initially reported eating "other deli meats," a category that would include hog head cheese. The traceback investigation determined that only one brand of hog head cheese was sold at both stores, suggesting that this brand was the outbreak source.
OPH sanitarians conducted an environmental investigation at both grocery stores to gather additional information on the suspect product. The sanitarians determined that hog head cheese offered for sale arrived in small, 0.7 pound blocks that were individually vacuum-sealed at the processing establishment. Each store weighed and priced the product and sold it in the refrigerated meat section. The sanitarians collected one unopened package of mild hog head cheese from the first store and two unopened packages of hog head cheese, one mild and one spicy, from the second store. At CDC's Enteric Diseases Laboratory Branch, L. monocytogenes serotype 1/2a with the outbreak PFGE pattern combination was isolated from the package of spicy hog head cheese.
This finding triggered a voluntary recall of approximately 500,000 pounds of hog head cheese and sausage that was processed on the same equipment. LDAF also collected 16 environmental samples from the processing establishment. Cultures of samples from a refrigeration unit and a door threshold yielded L. monocytogenes. An isolate from the refrigeration unit exhibited the outbreak PFGE pattern combination, and an isolate from the door threshold exhibited a pattern combination that was new to the PulseNet database (GX6A16.1362 and GX6A12.1939). CDC and the USDA Agricultural Research Service further characterized the patient, product, and environmental isolates using multiple-locus variable-number tandem repeat analysis and multilocus genotyping (3). All isolates, with the exception of the isolate from the door threshold, displayed indistinguishable multiple-locus variable-number tandem repeat analysis patterns and identical multilocus genotyping haplotypes (2.12_1/2a), further strengthening the association between the outbreak-associated cases and the hog head cheese producer.
Reported by
E Delaune, MPH, T Sokol, MPH, R Ratard, MD, Louisiana Office of Public Health. L Allen, MSPH, B Kissler, MPH, S Seys, MPH, K Holt, DVM, P Evans, PhD, Food Safety and Inspection Svc, T Ward, PhD, Agricultural Research Svc, US Dept of Agriculture; B Silk, PhD,* K Jackson, MPH, L Graves, E Trees, PhD, DVM, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC. *Corresponding contributor: Benjamin Silk, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC, 404-639-0536, bsilk@cdc.gov.
Editorial Note
L. monocytogenes can be found in soil, water, and silage, and causes a spectrum of illness ranging from asymptomatic infection to severe disease in both animals and humans. Invasive listeriosis, including sepsis and meningoencephalitis, occurs predominantly in older adults, persons with impaired immune systems, fetuses, and neonates. Based on its ubiquitous nature and the ability of the bacterium to establish itself in food processing environments, L. monocytogenes presents unique challenges for the food industry and regulatory agencies in their efforts to prevent the contamination of RTE foods. In addition, unlike most foodborne pathogens, L. monocytogenes can multiply at refrigerator temperatures.
Most cases of invasive listeriosis in the United States are sporadic (4). However, the advent of PulseNet for molecular subtyping of foodborne bacterial pathogens has revolutionized the ability of public health and regulatory officials to detect clusters and outbreaks and trace them to their sources (5). PulseNet is a network of laboratories in local, state, and federal health and regulatory agencies that use standard protocols, equipment, and nomenclature to upload PFGE patterns into a central database for comparison with one another. For L. monocytogenes, this usually consists of two patterns per isolate (i.e., images resulting from the use of two restriction enzymes, Ascl and Apal). In Louisiana, when OPH epidemiologists noted an unusually high listeriosis case count in 2010, PulseNet showed through molecular subtyping that eight cases were related, prompting the investigation.
Epidemiologic investigations of listeriosis clusters are challenging because case counts often are relatively small, some patients might not be available for interview, and others frequently report consumption of common food items that are higher-risk foods for L. monocytogenes contamination (6). In addition, the lengthy and variable incubation period of listeriosis (3--70 days) can result in recall bias and difficulty establishing an appropriate exposure period for food histories (7). Finally, immunocompromised persons who would be suitable controls for matched case-control studies often are difficult to identify. To address these challenges, CDC established the Listeria Initiative in 2004 to aid investigations of listeriosis clusters by using a standardized, extended case-form questionnaire to obtain timely food exposure histories from all persons with listeriosis reported in the United States (2). Patients are interviewed once illness is confirmed (rather than waiting for cluster detection). Using the Listeria Initiative questionnaire and associated database, hog head cheese was recognized as an uncommon food item that was common among the patients.
The implicated brand of hog head cheese originated from a small, state-inspected processing establishment in Louisiana, which produces approximately 600 pounds of hog head cheese per week. This establishment was under federal inspection until January 2007. Routine FSIS microbiologic testing of products at the establishment detected L. monocytogenes contamination in October and December 2006; the company voluntarily recalled 290 pounds of hog head cheese in January 2007. Four L. monocytogenes isolates from USDA-FSIS samples collected in 2006 did not match the 2010 outbreak-related PFGE pattern combination. In addition, Listeria contamination was not detected in any of the 12 product samples collected by LDAF since 2007; analysis of routine environmental samples collected by the management of the processing establishment during January--July 2010 also did not detect Listeria. However, the outbreak strain was identified in environmental samples collected during the investigation, which was several weeks after the manufacture of the outbreak-associated products (Figure), suggesting that persistent environmental contamination in the processing establishment was responsible for product contamination and resulting illnesses.
USDA-FSIS and state-inspected, meat-producing and poultry-producing establishments are required to develop a hazards analysis critical control points (HACCP) plan to prevent or eliminate reasonable hazards (including L. monocytogenes contamination of RTE products) using effective interventions. An FSIS risk assessment (8) determined that using combinations of interventions (e.g., testing and sanitation of food contact surfaces, prepackaging and postpackaging interventions, and the use of growth inhibitors) was more effective than any single intervention. The Listeria Rule† encourages establishments producing RTE products subject to postlethality contamination (e.g., contamination after cooking) to introduce combinations of interventions to eliminate and prevent the growth of L. monocytogenes in their products. Establishments choosing not to introduce such interventions or to only introduce growth inhibitors are required to test food contact surfaces for Listeria and are subject to more frequent product and surface sampling by the regulatory agency.
Although this is the first report of a listeriosis outbreak associated with the consumption of hog head cheese, RTE deli meats are a recognized vehicle for Listeria infection and have been associated with several past outbreaks in the United States (9). Persons at risk for listeriosis, including older adults, pregnant women, and persons with immunocompromising conditions or therapies, should take additional precautions to lower their risk for infection.§ CDC, USDA-FSIS, and FDA have developed food safety education guidance for persons at risk for listeriosis and those who prepare meals for at-risk persons (Box).
Acknowledgments
The findings in this report are based, in part, on the contributions of state and federal field staff members; W Glapion, M Walker, R Darville, A Bernard, T Jefferson, J Bailey, S Howat, Louisiana Office of Public Health; J Cornett, DVM, R Bane, E Thompson, A Busch, DVM, J Coleman, K Kenne, R Eckel, Food Safety and Inspection Svc, T Usgaard, Agricultural Research Svc, US Dept of Agriculture; and T Harper, A Sabol, TA Nguyen, MPH, I Williams, PhD, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC.
References
- US Department of Agriculture, Food Safety and Inspection Service. Compliance guidelines to control Listeria monocytogenes in post-lethality exposed ready-to-eat meat and poultry products. Washington, DC: US Department of Agriculture; 2006 Available at http://www.fsis.usda.gov/oppde/rdad/frpubs/97-013f/lm_rule_compliance_guidelines_may_2006.pdf. Accessed March 29, 2011.
- Council of State and Territorial Epidemiologists. CSTE position statement 03-ID-01: Listeria case surveillance. Atlanta, GA: Council of State and Territorial Epidemiologists; 2003. Available at http://www.cste.org/ps/2003pdfs/2003finalpdf/03-id-01revised.pdf. Accessed March 29, 2011.
- Ward TJ, Ducey TF, Usgaard T, Dunn KA, Bielawski JP. Multilocus genotyping assays for single nucleotide polymorphism-based subtyping of Listeria monocytogenes isolates. Appl Environ Microbiol 2008;74:7629--42.
- Varma JK, Samuel MC, Marcus R, et al. Listeria monocytogenes infection from foods prepared in a commercial establishment: a case-control study of potential sources of sporadic illness in the United States. Clin Infect Dis 2007;44:521--8.
- Graves LM, Hunter SB, Ong AR, et al. Microbiological aspects of the investigation that traced the 1998 outbreak of listeriosis in the United States to contaminated hot dogs and establishment of molecular subtyping-based surveillance for Listeria monocytogenes in the PulseNet network. J Clin Microbiol 2005;43:2350--5.
- Food and Drug Administration, US Department of Agriculture. Listeria monocytogenes risk assessment: quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. Silver Spring, MD: US Food and Drug Administration; 2003. Available at http://www.fda.gov/food/scienceresearch/researchareas/riskassessmentsafetyassessment/ucm183966.htm. Accessed April 6, 2011.
- Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect 2007;9:1236--43.
- US Department of Agriculture, Food Safety Inspection Service. Risk assessment for Listeria monocytogenes in deli meat. Washington, DC: US Department of Agriculture; 2003. Available at http://www.fsis.usda.gov/pdf/lm_deli_risk_assess_final_2003.pdf. Accessed March 29, 2011.
- Gottlieb SL, Newborn, EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis 2006;42:29--36.
* Available at http://www.cdc.gov/nationalsurveillance/listeria_surveillance.html.
† Additional information available at http://www.fsis.usda.gov/oppde/rdad/frpubs/97-013f.htm.
§ Additional information available at http://www.cdc.gov/nczved/divisions/dfbmd/diseases/listeriosis.
What is already known on this topic?
Multistate outbreaks of listeriosis led to U.S. regulatory policy changes and industry controls of Listeria monocytogenes contamination in ready-to-eat (RTE) meat and poultry products.
What is added by this report?
This is the first report of an association between an outbreak of invasive listeriosis and hog head cheese, indicating continuing challenges for RTE meat processors to prevent L. monocytogenes contamination, and the vulnerability of at-risk populations to invasive infections through consumption of contaminated RTE meat.
What are the implications for public health practice?
The combined application of PulseNet, a molecular subtyping network, and the Listeria Initiative, an enhanced surveillance program, was indispensible for the outbreak investigation and subsequent identification and recall of potentially contaminated product.
FIGURE. Number of invasive listeriosis cases, by month of patient specimen collection, and investigation timeline after an outbreak associated with consumption of hog head cheese --- Louisiana, 2010
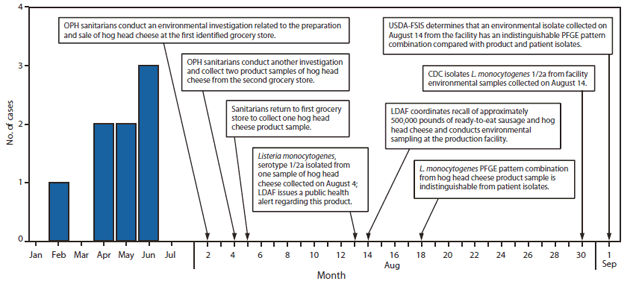
Abbreviations: OPH = Louisiana Office of Public Health; LDAF = Louisiana Department of Agriculture and Forestry; PFGE = pulsed-field gel electrophoresis; USDA-FSIS = U.S. Department of Agriculture Food Safety and Inspection Service.
Alternate Text: The figure above shows the number of invasive listeriosis cases, by month of patient specimen collection, and investigation timeline, after an outbreak associated with consumption of hog head cheese in Louisiana in 2010. Illness onsets occurred from February 18 to June 16.
|
BOX. Guidance for listeriosis prevention among persons at risk |
|---|
|
Eating food contaminated with the bacterium Listeria monocytogenes can cause a potentially life-threatening, invasive disease called listeriosis. Pregnant women, older adults, and persons with weakened immune systems caused by medical conditions or treatment are at higher risk for listeriosis. Symptoms include fever, headache, stiff neck, confusion, loss of balance, and convulsions. Pregnant women might experience only mild illness; however, listeriosis during pregnancy can lead to miscarriage or stillbirth, premature delivery, or life-threatening infection of the newborn. CDC, the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service, and Food and Drug Administration recommend that at-risk persons and those who prepare meals for at-risk persons adhere to the following guidance. Pay attention to the following foods and advice:
To keep food safe:
Follow these four simple steps:
Additional food safety guidance for at-risk persons and multi-language publications are available at http://origin-www.fsis.usda.gov/fact_sheets/at_risk_&_underserved_fact_sheets/index.asp. |
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


