FIGURE 1. Initial case review of perinatal hepatitis B virus infections identified through two reporting systems for infants born in the United States in 2005
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Assessing Completeness of Perinatal Hepatitis B Virus Infection Reporting Through Comparison of Immunization Program and Surveillance Data --- United States
In the United States, an estimated 24,000 women with hepatitis B virus (HBV) infection give birth each year (1). To prevent mother-to-child HBV transmission, the Advisory Committee on Immunization Practices (ACIP) recommends administering postexposure prophylaxis of hepatitis B vaccine (HepB) and hepatitis B immune globulin (HBIG) to infants born to HBV-infected women within 12 hours of delivery, followed by completion of the HepB series (2). In 1990, CDC established a national Perinatal Hepatitis B Prevention Program (PHBPP) to support federal immunization program grantees in performing this ACIP-recommended case management of infants born to HBV-infected women. Perinatal HBV infections currently are reported by state and local health departments to CDC through two parallel processes: by immunization programs as part of federal program grant reporting requirements and by communicable disease surveillance units as part of the National Notifiable Diseases Surveillance System (NNDSS). A review of perinatal HBV infection reporting for infants born in 2005 identified 68 cases reported by immunization programs and 47 cases reported by communicable disease surveillance units, resulting in a total of 73 unique cases, 42 (58%) of which were reported by both systems. Following investigation, data reconciliation, and additional NNDSS reporting, 78 unique cases were identified, 63 (84%) of which were reported by both systems. Improved information-sharing between immunization programs and communicable disease surveillance units of health departments is essential to ensure more complete identification, case management, and quantification of perinatal HBV infections. Accuracy and completeness of perinatal HBV infection reporting can help ensure and measure progress toward elimination of HBV transmission in the United States.
A case of perinatal HBV infection is defined as hepatitis B surface antigen (HBsAg) positivity in any infant aged 1--24 months born in the United States or its territories to an HBsAg-positive mother (3). Since 1995, PHBPP activities have been reported to CDC through the Annual Immunization Program Report. Since 2001, communicable disease surveillance units have reported individual perinatal HBV infection cases to CDC through NNDSS. During 2001--2004, an average of 38 perinatal HBV infection cases per year (range: 18--54) were reported through NNDSS; during the same period, an average of 90 cases per year (range: 77--102) were reported via the Annual Immunization Program Report.
To investigate this discordance, CDC reviewed all reports of perinatal HBV infections in the two systems for infants born in 2005. To identify the 2005 birth cohort, NNDSS data entries from the reporting period January 1, 2005--March 30, 2007, were compared with those reported in the Annual Immunization Program Report submitted in April 2007. Because reporting of perinatal HBV infections through the annual report is in aggregate numbers and not line-listed as it is through NNDSS, for this analysis CDC requested PHBPP coordinators to share NNDSS case numbers and demographic information on HBV-infected infants enumerated in the annual report. Cases reported through the annual report or through NNDSS were then compared using infant date of birth, NNDSS case number, sex, and race. PHBPP coordinators were informed of discrepancies and asked to work with communicable disease surveillance unit staff members responsible for NNDSS data entry to reexamine program reports and NNDSS reports of perinatal HBV infections to resolve discrepancies.
Initially, 61 perinatal HBV infection cases were identified through NNDSS, and 86 were identified through the annual report (Figure 1). Fourteen cases reported through NNDSS were excluded: 11 because of erroneous reporting (nine reports actually were maternal HBV infections and not infant HBV infections, one infant was not born in the United States, and one was a duplicate entry) and three because of insufficient data to verify the case. Eighteen cases reported through the annual report were excluded: 11 because of erroneous reporting (seven infants not born in 2005 and four not HBsAg-positive) and seven (8%) because of insufficient data to verify the case. Case matching was complicated by incorrect or missing key data elements, such as race (eight annual report cases and 16 NNDSS cases), sex (one annual report case), and date of birth (two NNDSS cases). Before case reconciliation, of the 73 unique cases reported by the two reporting systems, 42 (58%) were reported by both (Figure 2). Case reconciliation included obtaining more information on previously unverified cases, NNDSS reporting of cases originally reported only in the annual report, and 7 months of additional reporting to NNDSS. Following case reconciliation, 78 unique cases were identified across the two reporting systems, with 63 (84%) reported by both (Figure 3).
When asked to identify factors that influenced whether a case was reported by both systems, health department staff members identified good communication between PHBPP coordinators and communicable disease surveillance staff as an important determinant of case reporting. Of 37 immunization program grantees who responded, 28 (76%) indicated regular communication between persons responsible for NNDSS data entry and the PHBPP coordinator before finalizing NNDSS data; 31 (84%) indicated that the PHBPP coordinator communicated with persons responsible for NNDSS data entry before submission of the annual report. PHBPP staff members reported that some incorrect or missed reporting resulted from misunderstanding of the questions and because annual reports often were completed by a person other than the PHBPP coordinator. Incorrect NNDSS reporting included misclassifications of perinatal HBV infections as acute HBV or chronic HBV infections and misclassifications of maternal HBV infections as perinatal HBV infections.
Reported by
DM Roque, MD, Magee-Womens Hospital of Univ of Pittsburgh Medical Center, Pennsylvania. SA Wang, MD,* A Wasley, ScD, Global Immunization Div; L Jacques-Carroll, MA, S Roush, MPH, CM Weinbaum, MD, Immunization Svcs Div, National Center for Immunization and Respiratory Diseases, CDC. *Corresponding contributor: Susan Wang, Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC, +41 22 791 1606, sjw8@cdc.gov.
Editorial Note
Comparison of immunization program and communicable disease surveillance data for reported perinatal HBV infections demonstrates a need for improved quality and completeness of both reporting systems. Some infants were reported for the wrong calendar year in the annual report, whereas case definitions were misinterpreted (e.g., maternal infections were miscoded as perinatal infections) in NNDSS. In the two reporting systems, 5%--8% of cases contained insufficient data to verify cases, and 13%--18% of cases were erroneous reports.
At the local level, communicable disease surveillance staff members should ensure that every infant with perinatal HBV infection is reported to the PHBPP coordinator, so that immunization program staff can investigate whether failure to vaccinate (i.e., gaps in clinical or program services) or vaccine failure might have resulted in the perinatal HBV infection. In turn, PHBPP coordinators should ensure that every infant with perinatal HBV infection is reported to the communicable disease surveillance staff to ensure complete and accurate NNDSS surveillance data for monitoring disease, evaluating and improving prevention efforts, and allocating resources. Establishing joint cross-check procedures between immunization program and surveillance staff members, such as ensuring that each infected infant has a surveillance identification number, will help make sure that immunization programs and surveillance units have accurate and complete descriptions of all infants with perinatal HBV infection.
Although this analysis was performed to assess complete and accurate reporting of detected perinatal HBV infections for surveillance and program activities, increased case detection also is critically needed for achieving elimination of HBV transmission in the United States (2). Because HBV-infected infants are typically asymptomatic, case detection requires performing HBsAg testing of at-risk infants. However, because fewer than half the estimated number of births to HBV-infected women are identified through PHBPP (4) and only a fraction of those infants undergo postvaccination testing (1), the actual number of perinatal HBV infection cases is believed to be 10 to 20 times higher than the number currently detected and reported (1).
Whereas adults who acquire HBV infection have a 95% likelihood of resolving the infection and only a 5% risk for developing chronic HBV infection, infants who become HBV-infected have a 90% risk for developing chronic HBV infection and a 25% lifetime risk for dying prematurely from cirrhosis or hepatocellular carcinoma (1,2). Thus, the key strategy to eliminate morbidity and mortality from HBV is to prevent infants from acquiring HBV infection. ACIP recommends that all newborns receive their first HepB vaccination before hospital discharge (2). For infants born to HBV-infected women, administering ACIP-recommended postexposure prophylaxis of HBIG and HepB within hours of birth followed by completion of the HepB series has been shown to be 85%--95% effective in preventing HBV infection (2).
Multiple steps are involved in the prevention and monitoring of perinatal HBV infection, including 1) identifying HBV-infected pregnant women, 2) providing newborn infants with appropriate and timely postexposure prophylaxis, 3) monitoring infants born to infected women to ensure completion of the HepB series and to ensure HBsAg testing 1--2 months after the third HepB dose, and 4) reporting of any HBV infections among infants. Eliminating perinatal HBV transmission in the United States will require closing any gaps in this process (5). Accurate counting of HBV-infected infants is needed to monitor progress toward elimination of perinatal HBV infection, to know whether program and vaccine are reducing that burden effectively, and to identify and address any failures in program or vaccine when an infected infant is reported.
References
- Ward J. Time for renewed commitment to viral hepatitis prevention. Am J Public Health 2008;98:780--1.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: immunization of infants, children, and adolescents. MMWR 2005;54(No. RR-16).
- CDC. Hepatitis, viral, perinatal hepatitis B virus infection acquired in the United States or U.S. territories. Atlanta, GA: US Department of Health and Human Services, CDC; 1995. Available at http://www.cdc.gov/osels/ph_surveillance/nndss/casedef/hepatitisviralcurrent.htm. Accessed April 4, 2011.
- Din E, Wasley A, Jacques-Carroll L, Sirotkin B, Wang S. Estimating the number of births to hepatitis B virus-infected women in 22 states, 2006. Pediatric Infect Dis J 2011; January 24, 2011 [Epub ahead of print].
- Willis BC, Wortley P, Wang SA, Jacques-Carroll L, Zhang F. Gaps in hospital policies and practices to prevent perinatal transmission of hepatitis B virus in the United States. Pediatrics 2010;125:704--11.
What is already known on this topic?
In the United States, enumeration of nationally notifiable diseases occurs primarily through passive surveillance through the Nationally Notifiable Disease Surveillance System, and underreporting is common.
What is added by this report?
The existence of a National Perinatal Hepatitis B Prevention Program to support immunization programs in performing active identification of infants born to women infected by hepatitis B virus (HBV) provided an opportunity to compare the number of HBV-infected infants identified actively through the immunization programs with the number of HBV-infected infants identified passively through communicable disease surveillance. More infants were identified through active identification by the immunization programs than by passive communicable disease surveillance; however, gaps were observed in reporting by both programs and disease surveillance.
What are the implications for public health practice?
Greater coordination and communication between immunization program and communicable disease surveillance units in health departments is needed to ensure that all identified perinatal hepatitis B cases are reported and that all reported cases are followed up appropriately.
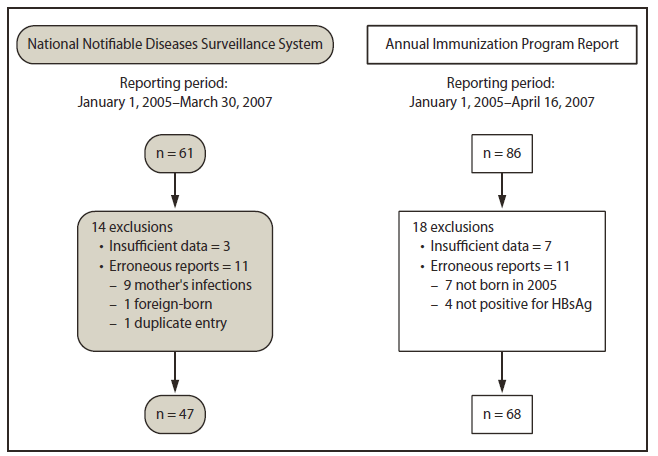
Abbreviation: HBsAg = hepatitis B surface antigen.
Alternate Text: The figure above shows the initial case review of perinatal hepatitis B virus infections identified through two reporting systems for infants born in the United States in 2005. Initially, 61 perinatal HBV infection cases were identified through NNDSS, and 86 were identified through the annual report.
FIGURE 2. Initial matching of cases of perinatal hepatitis B virus infection identified through two reporting systems for infants born in the United States in 2005
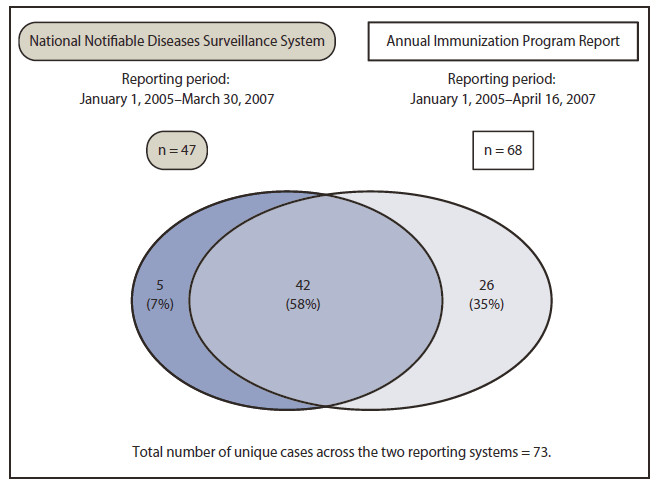
Alternate Text: The figure above shows initial matching of cases of perinatal hepatitis B virus infection identified through two reporting systems for infants born in the United States in 2005. Before case reconciliation, of the 73 unique cases reported by the two reporting systems, 42 (58%) were reported by both.
FIGURE 3. Second matching of cases of perinatal hepatitis B virus infection identified through two reporting systems for infants born in the United States in 2005, following efforts at data reconciliation and 7 months of additional reporting*
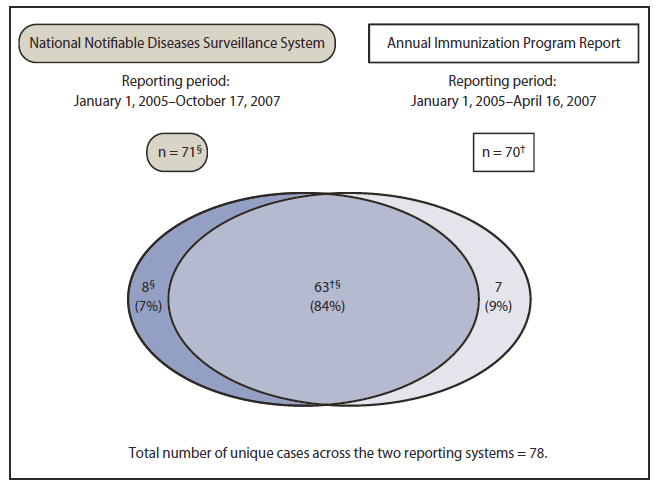
* Additional reporting through the National Notifiable Diseases Surveillance System (NNDSS) only.
† Includes two cases originally excluded from the annual report because of missing birth dates necessary to verify that the cases met the case definition; these cases subsequently were reported through NNDSS with the missing data included, allowing the cases to be matched across the two reporting systems.
§ Includes 24 additional cases reported to NNDSS in the 7 months of additional reporting. Of the 24 additional NNDSS cases, 21 matched annual report cases, and three cases were not captured in the annual report.
Alternate Text: The figure above shows second matching of cases of perinatal hepatitis B virus infection identified through two reporting systems for infants born in the United States in 2005, following efforts at data reconciliation and 7 months of additional reporting. Following case reconciliation, 78 unique cases were identified across the two reporting systems, with 63 (84%) reported by both.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


