FIGURE 1. Number* and percentage of respiratory specimens testing positive for influenza, by type, surveillance week, and year --- World Health Organization and National Respiratory and Enteric Virus Surveillance System collaborating laboratories, United States, October 3, 2010--May 21, 2011†
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Update: Influenza Activity --- United States, 2010--11 Season, and Composition of the 2011--12 Influenza Vaccine
During the 2010--11 influenza season, influenza activity* first began to increase in the southeastern United States, and peaked nationally in early February. Compared with the previous pandemic year (2009--10), higher rates of hospitalization were observed for persons aged ≥65 years during the 2010--11 season, whereas lower hospitalization rates were observed in younger populations than during the pandemic year. Overall, the percentages of outpatient visits for influenza-like illness (ILI) were lower during the 2010--11 season than the 2009--10 pandemic influenza season. In the United States, influenza A (H3N2) remained the predominant virus throughout the season; however, 2009 influenza A (H1N1) and influenza B viruses also circulated, and the predominant virus varied by U.S. Department of Health and Human Service (HHS) region and week. This report summarizes influenza activity in the United States during the 2010--11 influenza season (October 3, 2010--May 21, 2011) and describes the components of the 2011--12 Northern Hemisphere influenza vaccine.
Viral Surveillance
During October 3, 2010--May 21, 2011, World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories in the United States tested 246,128 specimens for influenza viruses; 54,226 (22%) were positive (Figure 1). Of the positive specimens, 40,282 (74%) were influenza A viruses, and 13,944 (26%) were influenza B viruses. Among the influenza A viruses, 28,545 (71%) were subtyped; 17,599(62%) were influenza A (H3N2) viruses, and 10,946 (38%) were 2009 influenza A (H1N1) viruses.
The proportion of specimens testing positive for influenza during the 2010--11 season first exceeded 10%, indicating higher levels of virus circulation, during the week ending November 27, 2010. The proportion peaked at 36% during the week ending February 5, 2011, and declined to <10% during the week ending April 16, 2011.
Although influenza A (H3N2) viruses predominated, 2009 influenza A (H1N1) and influenza B viruses also circulated widely. The relative proportion of each type and subtype of influenza virus varied by region and week. From early November though early December, influenza B viruses accounted for 40%--49% of influenza viruses reported nationally, with the largest numbers reported from the southeastern states (HHS Region 4).† Influenza B viruses were predominant in Region 4 from early November through late December. The proportion of 2009 influenza A (H1N1) viruses increased nationally, beginning in January, and peaked during the week ending February 20, 2011, when 49% of all subtyped influenza A viruses were 2009 influenza A (H1N1) viruses. Although during this time influenza A (H3N2) viruses still predominated nationally, 2009 influenza A (H1N1) predominated in five of the 10 regions (Regions 3, 4, 5, 8, and 9) for 5--7 consecutive weeks, ranging from the week ending January 15 to the week ending April 2, 2011.
Novel Influenza A Viruses
Five cases of human infection with a novel influenza A virus were reported during the 2010--11 influenza season from three states. All five cases were infected with swine-origin influenza A (H3N2) viruses. Two cases occurred in September (Pennsylvania and Wisconsin), one case in October (Pennsylvania), and two cases in November (Minnesota). Two of the five cases occurred in adults, and three occurred in children. Two of the five cases were hospitalized; all five have recovered fully from their illness. The two cases in Pennsylvania were not related. The cases in Wisconsin and Pennsylvania had direct contact with swine or lived in areas close to swine farms. The two cases from Minnesota occurred in a father (index case) and child. The father had a nasopharyngeal swab positive for swine-origin influenza A (H3N2) virus and had direct swine exposure 6 days before illness onset. The child, whose infection with influenza A (H3N2) virus was confirmed several weeks later by serologic testing, did not have direct swine exposure, and most likely acquired infection from close contact with her father. Other persons in the same household also had ILI during the same period, but serologic results were either negative or inconclusive.
Antigenic Characterization
Since October 1, 2010, CDC has antigenically characterized 2,494 influenza viruses submitted by U.S. laboratories. Those have included 613 2009 influenza A (H1N1) viruses, 1,139 influenza A (H3N2) viruses, and 742 influenza B viruses. Of the 613 2009 influenza H1N1 viruses tested, 612 (99.8%) were characterized as A/California/7/2009-like, the 2009 influenza A (H1N1) component of the 2010--11 influenza vaccine. One virus (0.2%) of the 613 tested showed reduced titers with antiserum produced against A/California/7/2009. Of the 1,139 influenza A (H3N2) viruses, 1,103 (96.8%) were characterized as A/Perth/16/2009-like, the influenza A (H3N2) component of the 2010--11 influenza vaccine for the Northern Hemisphere. Of the 1,139 tested, 36 (3.2%) showed reduced titers with antiserum produced against A/Perth/16/2009.
Of the 742 influenza B viruses tested, 699 (94%) belonged to the B/Victoria lineage and 698 (99.9%) of these were characterized to be B/Brisbane/60/2008-like, the influenza B vaccine component for the 2010--11 Northern Hemisphere influenza vaccine. One (0.1%) of the 699 viruses belonging to the B/Victoria lineage showed reduced titers with antisera produced against B/Brisbane/60/2008. Of the 742 viruses tested, 43 (5.8%) belonged to the B/Yamagata lineage.
Resistance to Antiviral Medications
Since October 1, 2010, a total of 5,758 influenza virus specimens have been tested for antiviral resistance. All 723 influenza B viruses tested were sensitive to both oseltamivir and zanamivir. Among the 806 influenza A (H3N2) viruses tested for resistance to oseltamivir, two (0.2%) were found to be resistant. All 784 influenza A (H3N2) viruses tested for resistance to zanamivir were sensitive. Among the 4,229 2009 influenza A (H1N1) viruses tested for resistance to oseltamivir, 39 (0.9%) were found to be resistant, and all of the 771 viruses tested for resistance to zanamivir were found to be sensitive. High levels of resistance to the adamantanes (i.e., amantadine and rimantadine) persist among 2009 influenza A (H1N1) and influenza A (H3N2) viruses currently circulating globally.
Composition of the 2011--12 Influenza Vaccine
The Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee recommended that the 2011--12 trivalent influenza vaccine for the United States contain A/California/7/2009-like (H1N1), A/Perth/16/2009-like (H3N2), and B/Brisbane/60/2008-like viruses. This represents no change to any of the three components from the 2010--11 influenza vaccine formulation used in the United States or from the current formulation of the 2011 Southern Hemisphere influenza vaccines. These recommendations were based on antigenic analyses of influenza viruses that circulated in the United States and worldwide during 2010--11, epidemiologic data, postvaccination serologic studies in humans, and the availability of candidate vaccine strains and reagents.
Outpatient Illness Surveillance
The weekly percentage of outpatient visits for ILI§ to the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) exceeded national baseline levels¶ (2.5%) during the weeks ending December 25, 2010, through March 19, 2011 (weeks 51--11), and peaked at 4.6% during the week ending February 19, 2011 (week 7) (Figure 2). During the two influenza seasons before the pandemic (2007--08 and 2008--09), the peak percentage of patient visits for ILI ranged from 3.5% to 6.0% and occurred during mid- to late February. During the 2009 pandemic, however, the percentage of patient visits for ILI peaked at 7.7% in late October (1). The peak percentage of outpatient visits for ILI varied in time by region. The percentage of outpatient visits for ILI peaked in Regions 3, 4, and 5 during the week ending February 5, 2011 (week 5), in Regions 6, 8, 9, and 10 during the week ending February 19, 2011 (week 7), and in Regions 1 and 7 during the week ending February 26, 2011 (week 8). The percentage of visits for ILI in Region 2 peaked during the week ending January 1, 2011 (week 52); however, a second peak of similar magnitude occurred during the week ending February 26, 2011 (week 8). The increase in the percentage of visits for ILI during the week ending January 1, 2011 (week 52), likely was influenced by a reduction in preventive health-care visits during the holiday season, as has occurred during previous seasons.
Outpatient data collected in ILINet are used to produce a measure of ILI activity.** The number of states experiencing high ILI activity peaked at 19 states during the week ending February 12, 2011 (week 6).
State-Specific Activity Levels
State and territorial epidemiologists report the geographic distribution of influenza in their state through a weekly influenza activity code.†† The geographic distribution of influenza activity was most extensive during the week ending February 26, 2011 (week 8), when 44 states reported widespread influenza activity and five states reported regional influenza activity. No states reported widespread influenza activity after the week ending April 9, 2011 (week 15). The peak number of states reporting widespread or regional activity during the previous three seasons has ranged from 49 to 50 states (CDC, unpublished data, 2011).
Influenza-Associated Hospitalization
CDC monitors hospitalizations associated with laboratory-confirmed influenza infections using the FluSurv-NET surveillance system. FluSurv-NET§§ is a population-based surveillance network comprised of 10 surveillance sites of the Emerging Infections Program (EIP) and six sites that were added during the 2009--10 influenza season. Based on FluSurv-NET surveillance data, the cumulative hospitalization rate (per 100,000 population) for October 1, 2010--April 30, 2011, was 43.8 among children aged 0--4 years, 8.5 among children aged 5--17 years, 10.7 among adults aged 18--49 years, 21.7 among adults aged 50--64 years, and 62.5 among adults aged ≥65 years. The cumulative incidence for all age groups since October 1, 2010, was 20.5 per 100,000 (Figure 3). Based on EIP data alone, the cumulative hospitalization rate (per 100,000) for October 3, 2010--April 30, 2011, was 36.5 among children aged 0--4 years, 7.6 among children aged 5--17 years, 9.8 among adults aged 18--49 years, 20.3 among adults aged 50--64 years, and 62.1 among adults aged ≥65 years. The cumulative incidence for all age groups since October 1, 2010, was 19.1 per 100,000. During the past three seasons, rates for EIP sites have ranged from 34.6--67.3 per 100,000 for children aged ≤4 years, 5.8--25.2 for children aged 5--17 years, 3.6--24.4 for adults aged 18--49 years, 4.8--32.1 for adults aged 50--64, and 13.5--75.1 for adults aged ≥65 years.
As of April 30, 2011, among the 3,431 FluSurv-NET adult patients for whom medical chart data were available, the most frequent underlying conditions were cardiovascular disease (36%), metabolic disorders (35%), and chronic lung disease (23%). Among 1,008 children hospitalized with laboratory-confirmed influenza, 52% did not have any underlying conditions, and 19% had underlying asthma or reactive airway disease.
In April 2009, in response to the emergence of the 2009 influenza A (H1N1) virus, the Council of State and Territorial Epidemiologists (CSTE) initiated reporting of influenza-associated hospitalizations and deaths to CDC. On August 30, 2009, CDC and CSTE instituted modified case definitions for aggregate reporting of influenza-associated hospitalizations and deaths. This cumulative jurisdiction-level reporting is referred to the Aggregate Hospitalization and Death Reporting Activity (AHDRA) surveillance system. From October 3, 2010 to May 21, 2011, a total of 16,410 laboratory-confirmed, influenza-associated hospitalizations were reported to CDC. The median number of jurisdictions reporting hospitalizations per week through AHDRA was 22 (range: 13--24). The number of hospitalizations peaked during the week ending February 26, 2011 (week 8).
Pneumonia- and Influenza-Related Mortality
During the 2010--11 influenza season, the percentage of deaths attributed to pneumonia and influenza (P&I) exceeded the epidemic threshold¶¶ for 13 consecutive weeks, from the weeks ending January 29 to April 23, 2011 (weeks 4--16) (Figure 4). The percentage of deaths attributed to P&I peaked at 8.9% during the week ending February 12, 2011 (week 6). From the 2007--08 season through the 2009--10 season, the peak percentage of deaths attributed to P&I ranged from 8.0% to 9.1% and the total number of consecutive weeks above the epidemic threshold ranged from one to 11 (CDC, unpublished data, 2011).
From October 3, 2010, to May 21, 2011, a total of 311 laboratory-confirmed, influenza-associated deaths were reported to CDC through AHDRA. The mean number of jurisdictions reporting influenza-associated deaths per week through AHDRA was 21 (range: 12--23). The number of deaths peaked during the week ending March 12, 2011 (week 10).
Influenza-Related Pediatric Mortality
From October 3, 2010, to May 21, 2011, 105 laboratory-confirmed influenza-associated pediatric deaths were reported. These deaths were reported from 33 states,*** Chicago, and New York City. The mean and median ages of children who died were 7.1 years and 5.8 years, respectively; 15 children were aged <6 months, 14 were aged 6--23 months, 17 were aged 2--4 years, 30 were aged 5--11 years, and 29 were aged 12--17 years. Of the 105 deaths, 40 were associated with influenza B viruses, 27 with 2009 influenza A (H1N1) virus, 18 with influenza A (H3N2) viruses, and 20 with influenza A virus for which the subtype was not determined. For comparison, during the 2009--10 pandemic, 348 pediatric deaths were reported during April 15, 2009--October 2, 2010. Before the pandemic, 67 influenza-associated pediatric deaths were reported for the 2008--09 season, and 88 deaths were reported for the 2007--08 season.
Reported by
World Health Organization Collaborating Center for Surveillance, Epidemiology, and Control of Influenza. Krista Kniss, MPH, Scott Epperson, MPH, Lenee Blanton, MPH, Desiree Mustaquim, MPH, Amber Bishop, MPH, Tiffany D'Mello, MPH, Alejandro Perez, MPH, Rosaline Dhara, MPH, Lynnette Brammer, MPH, Larisa Gubareva, MD, Teresa Wallis, MS, Xiyan Xu, MD, Joseph Bresee, MD, Alexander Klimov, PhD, Nancy Cox, PhD, Lyn Finelli, DrPH, Influenza Div, National Center for Immunization and Respiratory Diseases, CDC. Corresponding contributor: Krista Kniss, kkniss@cdc.gov, 404-639-3747.
Editorial Note
During the 2010--11 season, influenza activity peaked in early February, and influenza A (H3N2) viruses predominated. The proportions of influenza viruses varied by region and by week. The proportion of influenza B viruses reported was highest early in the season, with the majority of these viruses reported from the southeastern states, and 2009 influenza A (H1N1) viruses became the most common in several regions in the later part of the season. Almost all influenza viruses sent to CDC for further characterization were antigenically similar to one of the components of the 2010--11 Northern Hemisphere vaccine.
In comparison with the past three seasons, the 2010--11 influenza season was less severe than the pandemic year (2009--10) and the 2007--08 season, but more severe than the 2008--09 influenza season, as determined by the percentage of deaths resulting from pneumonia or influenza, the number of influenza-associated pediatric deaths reported, and the percentage of visits to outpatient clinics for ILI. However, all age groups were affected substantially during the 2010--11 season because of widespread cocirculation of influenza A (H3N2), 2009 influenza A (H1N1), and influenza B viruses. Hospitalization rates overall were similar to rates reported during the 2007--08 influenza season, when influenza A (H3N2) was the predominant strain. Cumulative hospitalization rates reported for the 2010--11 season for persons aged <65 years were lower than rates reported during the pandemic, but the rates reported in these age groups were higher than those reported during the 2007--08 season. However, the cumulative hospitalization rate for persons aged ≥65 years was lower than during the 2007--08 season, but higher than the rate reported during the pandemic.
Testing for seasonal influenza and monitoring for novel influenza virus infections should continue year-round, as should specimen submission to CDC for further antigenic and genetic analysis and antiviral resistance monitoring. The detection of five novel influenza cases of swine-origin influenza A (H3N2) infections since September 2010 further emphasizes the importance of continued surveillance for novel influenza strains. Although summer influenza activity in the United States typically is low, cases of influenza and even sporadic outbreaks commonly are detected in the United States throughout the summer. Health-care providers should remain vigilant and consider influenza as a potential cause of summer respiratory illnesses. Public health laboratories should send to CDC virus samples that they cannot type or subtype using standard methods immediately and submit isolates that otherwise are unusual, including all summer isolates, as soon as possible after identification.
Since 2010, CDC has recommended that all persons aged ≥6 months get an influenza vaccine yearly, preferably in the fall, before the U.S. influenza season begins (2). During other times of the year, persons traveling to parts of the world where influenza activity is ongoing, and who have not received the vaccine for the current season, should receive an influenza vaccine to protect themselves while traveling. This is particularly important for persons at high risk for influenza-related complications. This recommendation also applies to persons traveling within the temperate regions of the Northern Hemisphere as part of large tourist groups (e.g., on cruise ships) that might include persons from parts of the world where influenza activity is ongoing (3).
Persons should be vaccinated 2 weeks before travel, because it takes 2 weeks for vaccine-induced immunity to develop. Travelers also should be aware that all influenza vaccine manufactured for the 2010--11 season expires in the early summer of 2011, after which influenza vaccines will not be available in the United States until the fall, when the 2011--12 vaccine becomes available.
Antiviral drugs are an important adjunct to influenza vaccination for reducing illness severity. Based on new recommendations from the Advisory Committee on Immunization Practices released January 21, 2011, antiviral treatment is recommended as soon as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at higher risk for influenza-related complications (4).††† Antiviral treatment also may be considered for outpatients with confirmed or suspected influenza who do not have known risk factors for severe illness if treatment can be initiated within 48 hours of illness onset. Recommended antiviral medications include oseltamivir and zanamivir. Recent viral surveillance and resistance data indicate that >99% of currently circulating influenza virus strains are sensitive to these medications. Amantadine and rimantadine should not be used because of the sustained high levels of resistance to these drugs among circulating influenza A viruses.
Acknowledgments
Participating state health departments, territorial health departments, state public health laboratories. US WHO collaborating laboratories. National Respiratory and Enteric Virus Surveillance System collaborating laboratories. US Outpatient ILI Surveillance Network. FluSurv-NET. Aggregate Hospitalization and Death Reporting Activity. Influenza Associated Pediatric Mortality Surveillance System. 122 Cities Mortality Reporting System. WHO FluNet.
References
- Brammer L, Blanton L, Epperson S, et al. Surveillance for influenza during the 2009 influenza A (H1N1) pandemic---United States, April 2009--March 2010. Clin Infect Dis 2011;52(Suppl 1):S27--35.
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-8).
- CDC. Influenza prevention: information for travelers. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at http://www.cdc.gov/flu/travelers/travelersfacts.htm. Accessed May 26, 2011.
- CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-1).
* The CDC influenza surveillance system collects five categories of information from 10 data sources: 1) viral surveillance (World Health Organization collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System, and novel influenza A virus case reporting); 2) outpatient illness surveillance (U.S. Outpatient Influenza-like Illness Surveillance Network); 3) mortality (122 Cities Mortality Reporting System, Aggregate Hospitalization and Death Reporting Activity, and influenza-associated pediatric mortality reports); 4) hospitalizations (FluSurv-NET, which includes the Emerging Infections Program and surveillance in six additional states, and Aggregate Hospitalization and Death Reporting Activity); and 5) summary of the geographic spread of influenza (state and territorial epidemiologist reports).
† The 10 HHS regions include the following states and territories: Region 1: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont; Region 2: New Jersey, New York, Puerto Rico, and the U.S. Virgin Islands; Region 3: Delaware, District of Columbia, Maryland, Pennsylvania, Virginia, and West Virginia; Region 4: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee; Region 5: Illinois, Indiana, Michigan, Minnesota, Ohio, and Wisconsin; Region 6: Arkansas, Louisiana, New Mexico, Oklahoma, and Texas; Region 7: Iowa, Kansas, Missouri, and Nebraska; Region 8: Colorado, Montana, North Dakota, South Dakota, Utah, and Wyoming; Region 9: Arizona, California, Hawaii, Nevada, American Samoa, Commonwealth of the Northern Mariana Islands, Federated States of Micronesia, Guam, Marshall Islands, and Republic of Palau; Region 10: Alaska, Idaho, Oregon, and Washington.
§ Defined as a temperature ≥100.0°F (≥37.8°C), oral or equivalent, and cough or sore throat, in the absence of a known cause other than influenza.
¶ The national and regional baselines are the mean percentage of visits for ILI during noninfluenza weeks for the previous three seasons plus two standard deviations. A noninfluenza week is a week during which <10% of specimens tested positive for influenza. National and regional percentages of patient visits for ILI are weighted on the basis of state population. Use of the national baseline for regional data is not appropriate.
** Activity levels are based on the percentage of outpatient visits in a state attributed to ILI and are compared with the average percentage of ILI visits that occur during spring and fall weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being at or below the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than the average. Because the clinical definition of ILI is nonspecific, not all ILI is caused by influenza; however, when combined with laboratory data, the information on ILI activity provides a useful picture of influenza activity in the United States.
†† Levels of activity are 1) no activity; 2) sporadic: isolated laboratory-confirmed influenza cases or a laboratory-confirmed outbreak in one institution, with no increase in activity; 3) local: increased ILI, or at least two institutional outbreaks (ILI or laboratory-confirmed influenza) in one region of the state, with recent laboratory evidence of influenza in that region; virus activity no greater than sporadic in other regions; 4) regional: increased ILI activity or institutional outbreaks (ILI or laboratory-confirmed influenza) in at least two but less than half of the regions in the state with recent laboratory evidence of influenza in those regions; and 5) widespread: increased ILI activity or institutional outbreaks (ILI or laboratory-confirmed influenza) in at least half the regions in the state, with recent laboratory evidence of influenza in the state.
§§ FluSurv-NET conducts population-based surveillance at sites in 10 Emerging Infections Program (EIP) states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee), and at sites in Idaho, Michigan, Ohio, Oklahoma, Rhode Island, and Utah.
¶¶ The seasonal baseline proportion of P&I deaths is projected using a robust regression procedure in which a periodic regression model is applied to the observed percentage of deaths from P&I that were reported by the 122 Cities Mortality Reporting System during the preceding 5 years. The epidemic threshold is set at 1.645 standard deviations above the seasonal baseline.
*** Arizona, California, Colorado, Florida, Georgia, Hawaii, Illinois, Indiana, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, North Carolina, North Dakota, New Jersey, New Mexico, Nevada, Ohio, Oklahoma, Oregon, Pennsylvania, South Dakota, Texas, Utah, Virginia, Vermont, Washington, West Virginia, and Wisconsin.
††† Persons at higher risk include children aged <5 years (especially those aged <2 years); adults aged ≥65 years; persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematologic (including sickle cell disease), metabolic disorders (including diabetes mellitus), or neurologic and neurodevelopment conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle, such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability [mental retardation], moderate to severe developmental delay, muscular dystrophy, or spinal cord injury); persons with immunosuppression, including that caused by medications or by human immunodeficiency virus infection; women who are pregnant or postpartum (within 2 weeks after delivery); persons aged ≤18 years who are receiving long-term aspirin therapy; American Indians/Alaska Natives; persons who are morbidly obese (i.e., body mass index ≥40); and residents of nursing homes and other chronic-care facilities.
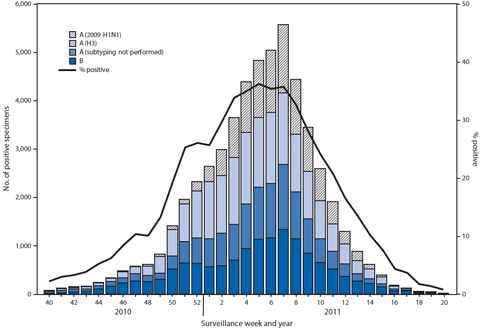
* N = 54,226.
† As of May 25, 2011.
Alternate Text: The figure above shows the number and percentage of respiratory specimens testing positive for influenza by type, surveillance week, and year in the United States from October 3, 2010-May 21, 2011, according to the World Health Organization and National Respiratory and Enteric Virus Surveillance System collaborating laboratories. During October 3, 2010 - May 21, 2011, World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laborato¬ries in the United States tested 246,128 specimens for influenza viruses; 54,226 (22%) were positive.
FIGURE 2. Percentage of outpatient visits for influenza-like illness (ILI) reported, by surveillance week and year --- U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet), United States, September 30, 2007--May 21, 2011*
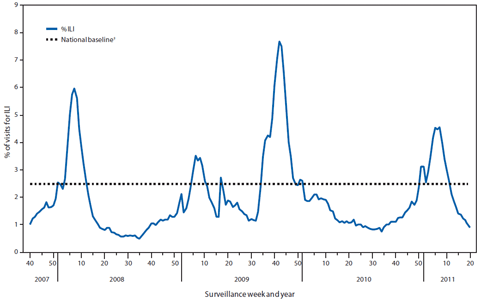
* As of May 25, 2010.
† The national and regional baselines are the mean percentage of visits for ILI during noninfluenza weeks for the previous three seasons, plus two standard deviations. A noninfluenza week is a week during which <10% of specimens tested positive for influenza. National and regional percentages of patient visits for ILI are weighted on the basis of state population. Use of the national baseline for regional data is not appropriate.
Alternate Text: The figure above shows the percentage of outpatient visits for influenza-like illness (ILI) reported, by surveillance week and year in the United States from September 30, 2007-May 21, 2011, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). The weekly percentage of outpatient visits for ILI to the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) exceeded national baseline levels (2.5%) during the weeks ending December 25, 2010, through March 19, 2011 (weeks 51-11), and peaked at 4.6% during the week ending February 19, 2011 (week 7).
FIGURE 3. Cumulative rate of laboratory-confirmed influenza-associated hospitalizations, by age group, surveillance week, and year --- FluSurv-NET,* United States, October 1, 2010--April 30, 2011
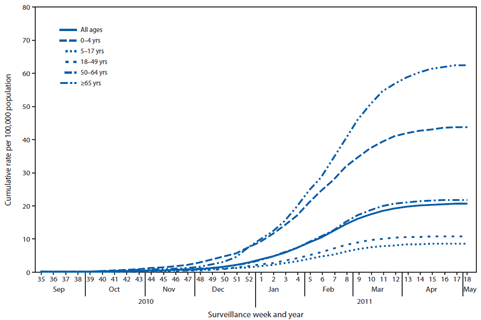
* FluSurv-NET results include surveillance at Emerging Infections Program sites and at sites in six additional states (Idaho, Michigan, Ohio, Oklahoma, Rhode Island, and Utah). Rates are based on 5,968 total cases for the period, of which 898 occurred among persons aged 0--4 years, 423 among persons aged 5--17 years, 1,453 among persons aged 18--49 years, 1,146 among persons aged 50--64 years, and 2,048 among persons aged ≥65 years.
Alternate Text: The figure above shows the cumulative rate of laboratory-confirmed influenza-associated hospitalizations, by age group, surveillance week, and year in the United States from October 1, 2010-April 30, 2011, according to FluSurv-NET. The cumulative incidence for all age groups since October 1, 2010, was 20.5 per 100,000.
FIGURE 4. Percentage of all deaths attributed to pneumonia and influenza (P&I), by surveillance week and year ---122 Cities Mortality Reporting System, United States, 2006--2011
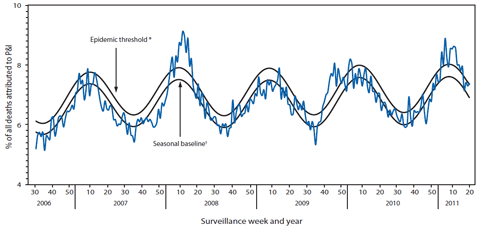
* The epidemic threshold is1.645 standard deviations above the seasonal baseline.
† The seasonal baseline is projected using a robust regression procedure that applies a periodic regression model to the observed percentage of deaths from P&I during the preceding 5 years.
Alternate Text: The figure above shows the percentage of all deaths attributed to pneumonia and influenza (P&I) by surveillance week and year in the United States from 2006-2011, according to the Mortality Reporting Systems of 122 cities. During the 2010-11 influenza season, the percentage of deaths attributed to P&I exceeded the epidemic threshold for 13 consecutive weeks, from the weeks ending January 29 to April 23, 2011 (weeks 4-16).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


