Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Lead Poisoning in Pregnant Women Who Used Ayurvedic Medications from India — New York City, 2011–2012
Lead poisoning still occurs in the United States despite extensive prevention efforts and strict regulations. Exposure to lead can damage the brain, kidneys, and nervous and reproductive systems. Fetal exposure to lead can adversely affect neurodevelopment, decrease fetal growth, and increase the risk for premature birth and miscarriage (1). During 2011–2012, the New York City Department of Health and Mental Hygiene (DOHMH) investigated six cases of lead poisoning associated with the use of 10 oral Ayurvedic medications made in India. All six cases were in foreign-born pregnant women assessed for lead exposure risk by health-care providers during prenatal visits, as required by New York state law. Their blood lead levels (BLLs) ranged from 16 to 64 µg/dL. Lead concentrations of the medications were as high as 2.4%; several medications also contained mercury or arsenic, which also can have adverse health effects. DOHMH distributed information about the medications to health-care providers, product manufacturers, and government agencies in the United States and abroad, via postal and electronic mail. DOHMH also ordered a local business selling contaminated products to cease sales. Health-care providers should ask patients, especially foreign-born or pregnant patients, about any use of foreign health products, supplements, and remedies such as Ayurvedic medications. Public health professionals should consider these types of products when investigating heavy metal exposures and raise awareness among health-care providers and the public regarding the health risks posed by such products.
The six patients in this report all were asymptomatic pregnant women whose health-care providers assessed them to be at risk for lead exposure. New York state law requires assessment of patients for risk of lead exposure during the first prenatal visit and testing of those determined to be at risk; CDC also recommends routine testing of pregnant women from at-risk populations (e.g., recent immigrants and women who use traditional remedies) (1). The New York State Department of Health forwards all blood lead test results from New York City residents to DOHMH, which conducts follow-up interviews and case investigations for adults identified with BLLs ≥10 µg/dL. Identification and removal of the lead source is the main priority. Women in the second half of pregnancy with BLLs 45–69 µg/dL are considered for chelation therapy. Pregnant women with BLLs ≥70 µg/dL are considered for chelation regardless of trimester. Pregnant women with lead encephalopathy should receive chelation regardless of trimester (1).
During 2004–2012, through case investigations and agency sweeps of local stores triggered by investigations or published reports, DOHMH identified 22 oral medications, supplements, or remedies containing high levels of heavy metals (Table). Twenty of the 22 products were brought into the United States (one product lacked country of origin information). DOHMH identified 10 of these 22 products during investigations of the six pregnant women with lead poisoning described in this report.
Case Reports
Patient 1. In January 2011, a woman born in India, aged 30 years, had a BLL of 64 µg/dL during week 27 of pregnancy. The woman took 1–2 capsules daily for 4 months of Pregnita, an Ayurvedic medication manufactured and purchased in India. She had obtained Pregnita from a practitioner in India who prescribed it for pregnancy-related nausea and vomiting. Testing found Pregnita contained 1.2% lead. Based on her reported use, the woman had consumed approximately 9–18 mg of lead daily, or 1.1–2.2 g of lead over the 4 months. The woman was hospitalized and received chelation therapy with calcium disodium ethylenediaminetetraacetic acid. Her BLL decreased to 36 µg/dL 5 days after chelation and to 20 µg/dL 3 months later (2 weeks after delivering). Her newborn's BLL was 23 µg/dL at 3 days after birth.
Patient 2. In May 2011, a woman born in Colombia, aged 36 years, had a BLL of 16 µg/dL reported during week 5 of pregnancy. She had used two Ayurvedic medications manufactured in India for skin problems (1 tablet of each daily) approximately 1–2 months before pregnancy and sporadically used the medications during the first month of pregnancy. She reported purchasing the medications, which were made in India, in New York City. Vatvidhwansan Ras (Figure) contained 2% lead, 1.5% mercury and 130 parts per million (ppm) arsenic. Kankayan Bati (Gulma) contained 12 ppm lead, 35 ppm mercury, and 9.5 ppm arsenic. Although difficult to ascertain exposure, if the woman ingested 1 tablet daily of each pill for 3 months, she would have consumed approximately 3 mg of lead daily, or 270 mg of lead during the entire period. In July, after discontinuing use, her BLL decreased to 10 µg/dL. In November, 3 months before delivery, her BLL was 1 µg/dL. The newborn's BLL was not measured.
Patient 3. In June 2011, a woman born in India, aged 24 years, had a BLL of 49 µg/dL reported during week 15 of pregnancy. She ingested two tablets of the Ayurvedic prenatal medication Garbhapal Ras daily to "keep her pregnancy and fetus healthy." She started use at approximately week 9 of pregnancy and continued for about 6 weeks. Her father, an Ayurvedic practitioner in India, prescribed and mailed the medication to her in an unlabeled container. Garbhapal Ras was found to contain 2.2% lead. Based on her reported use, the woman had consumed approximately 7 mg of lead daily or 300 mg of lead over the 6-week period. The product also was found to contain 1.9% mercury and 410 ppm arsenic. Seven weeks later, after discontinuing use, the woman's BLL decreased to 26 µg/dL. Her newborn's BLL was 7 µg/dL at birth.
Patient 4. In August 2011, a woman born in India, aged 35 years, had a BLL of 42 µg/dL reported during week 8 of pregnancy. She had a history of miscarriages and used four Ayurvedic medications approximately 2 months before pregnancy to promote fertility. She ceased use upon learning she was pregnant. She had obtained the medications while in India from an Ayurvedic practitioner. One of the medications, Ovarin (Figure), was found to contain as much as 1.2% lead, 1,000 ppm arsenic, and 1.8% mercury, and the woman reported ingesting 1–2 capsules of Ovarin daily. Based on her reported use, the woman had consumed approximately 6–12 mg of lead daily, or 360–720 mg of lead during the 2 months. She miscarried at approximately 11 weeks' gestation.
Patient 5. In January 2012, a woman born in India, aged 33 years, had a BLL of 52 µg/dL reported during week 10 of pregnancy. She began using five different Ayurvedic medications to improve fertility and one to improve skin complexion about 7 months before her pregnancy. She used each product once or twice daily for approximately 4 months. An Ayurvedic practitioner had provided her with the medications during a previous visit to India. Elevated levels of lead, mercury, or arsenic were found in five of the six medications. Ovarin was found to contain 2.4% lead, 7% mercury, and 100 ppm arsenic. Garbha Dharak Yog was found to contain 10% mercury, 140 ppm arsenic, and 110 ppm lead. Laxmana Louh was found to contain 180 ppm lead, 120 ppm mercury, and 12 ppm arsenic. Garbha Chintamani Ras (Vrihat) (Swarna Yukt) was found to contain 5.2% arsenic and 120 ppm lead. Pigmento was found to contain 2.9% mercury, 27 ppm arsenic, and 7.3 ppm lead. Based on her reported use, the woman had consumed approximately 12–24 mg of lead daily, or 1.4–2.9 g of lead during the 4 months. She miscarried during week 12 of pregnancy.
Patient 6. In May 2012, a woman born in India, aged 35 years, had a BLL of 24 µg/dL reported during week 22 of pregnancy. In January, she had begun using six medications to increase her chances of "having a male baby." She obtained the medications from her mother-in-law, who visited an Ayurvedic practitioner in India on her behalf. She took the medication 1–3 times a day until she discontinued use in June. One of the medications, Garbhapal Ras, was found to contain 1.5% lead, 0.44% mercury, and 81 ppm arsenic. Based on her reported use, the woman had consumed approximately 2–7 mg of lead daily, or 300–1,000 mg over the 5 months. Her BLL decreased to 11 µg/dL 5 weeks after discontinuing the medications. The woman had not yet delivered as of August 20.
Reported by
Paromita Hore, PhD, Munerah Ahmed, MPH, Jacqueline Ehrlich, MD, Celia Ng, MSN, Lourdes Steffen, Slavenka Sedlar, MA, Phyllis Curry-Johnson, EdD, Nathan Graber, MD, Deborah Nagin, MS, Nancy Clark, MA, Bur of Environmental Disease Prevention, New York City Dept of Health and Mental Hygiene. Robert Saper, MD, Boston Univ, Massachusetts. Marissa Scalia Sucosky, MPH, Div of Emergency and Environmental Health Svcs, National Center for Environmental Health, CDC. Corresponding contributor: Paromita Hore, phore@health.nyc.gov, 347-396-4177.
Editorial Note
Foreign-born pregnant women might be at increased risk for lead poisoning. Reasons include use of certain foreign products and increased bone stores of lead from past exposures. The body's demand for calcium increases during pregnancy to support fetal bone development, which might release these bone stores. In 2011, of the 205 New York City women reported to DOHMH with BLLs ≥10 µg/dL, 118 (58%) were pregnant, and 98 (83%) of the pregnant women were foreign-born (New York City Department of Health and Mental Hygiene, unpublished data, 2011). More than 70% of pregnant women with elevated BLLs interviewed by DOHMH in 2011 reported using foreign traditional or familiar products from their ancestral countries, such as cosmetics, medications, remedies, food, and pottery, suggesting that health-care providers should question pregnant women about their use of such products.
Pregnant women present a unique concern, because lead exposure can adversely affect the health of both mother and child. Fetal lead exposure increases the risks for low birth weight, developmental delay, reduced intelligence, and behavioral problems (1). Pregnant women exposed to lead might be at increased risk for gestational hypertension and spontaneous abortion (1). Exposure to other heavy metals, such as arsenic and mercury, also can have adverse health effects. Two of the six patients miscarried before 20 weeks' gestation. Both patients were taking Ayurvedic medications to promote fertility, and it is unknown whether underlying reproductive problems or heavy metal exposures contributed to the miscarriages.
Numerous cases of heavy metal poisonings associated with the use of foreign medications, supplements, traditional remedies, or other health products have been documented (2–5). In one study, 20% of South Asian herbal medications purchased in Boston-area stores contained heavy metals (6). Heavy metals might not be listed as ingredients and might only be identified by testing. Some heavy metal inclusion might result from incidental contamination during production (e.g., the use of contaminated raw ingredients or poor manufacturing equipment), whereas other inclusion might be intentional for perceived therapeutic benefits.
The cases of lead poisoning described in this report were associated with the use of Ayurvedic medications. Ayurveda is a millennia-old medical system closely connected to traditional culture and religion in India (7,8). According to a national survey, an estimated 214,000 adults in the United States visited an Ayurvedic practitioner in 2007, an increase of 39% since 2002 (8). Most Ayurvedic medications are marketed either as dietary supplements or for drug uses not approved by the Food and Drug Administration (FDA). None of the nine medications with labeling information that were used by the patients in this report has been the subject of an FDA import alert.* However, in a 2008 update, FDA urged consumers to use caution with Ayurvedic products.† Although not all Ayurvedic medications include heavy metals intentionally, all six patients in this report used "rasa shastra" medications. Rasa shastra is a type of Ayurvedic medication that is intentionally prepared with metal, mineral, or gem compounds (9). These compounds, called "bhasmas," sometimes are indicated on product labels.
DOHMH visits local stores to assess availability of products identified through case investigations and published reports or to collect and test products that are suspect. Stores selling contaminated products, such as the local business that sold medications to patient 2, are prohibited from any further sales of identified products and are ordered to return remaining stock to distributors. DOHMH also alerts local health-care providers through its Health Alert Network and notifies manufacturers. DOHMH reports contaminated products to the FDA dietary supplements adverse event reporting website§ and appropriate foreign authorities. Reporting to FDA is important to systematically gather data and understand the scope of the problem. Information regarding these products is forwarded to FDA global offices to encourage collaborative efforts to improve product safety in the United States and abroad.
The cases of lead poisoning among the six pregnant women underscore the importance of risk assessment for lead exposure and blood lead testing in at-risk populations. Health-care providers should 1) be aware that users might not readily disclose use of health products; 2) ask patients about their use of prescription and nonprescription medications and supplements, including Ayurvedic medications and other traditional remedies; 3) advise patients to stop using suspect products; and 4) consider testing patients for exposure to lead or other heavy metals if use is reported. Public health workers and health-care providers should consider the use of foreign supplements, medications, traditional remedies, or other health products as potential risk factors when investigating lead and other heavy metal poisonings, especially in foreign-born populations, and particularly among pregnant women. Risk assessments and testing conducted during prenatal visits are critical to identifying and intervening in heavy metal poisoning cases. Public health measures, such as blood lead testing and surveillance in the United States and elsewhere, are necessary to assess the extent of lead exposure and develop appropriate interventions.
References
- CDC. Guidelines for the identification and management of lead exposure in pregnant and lactating women. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Available at http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf. Accessed August 20, 2012.
- CDC. Lead poisoning associated with Ayurvedic medications—five states, 2000–2003. MMWR 2004;53:582–4.
- Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci 2002;23:136–9.
- Baer RD, Ackerman A. Toxic Mexican folk remedies for the treatment of empacho: the case of azarcon, greta, and albayalde. J Ethnopharmacol 1988;24:31–9.
- Al Khayat A, Menon NS, Alidini MR. Acute lead encephalopathy in early infancy—clinical presentation and outcome. Ann Trop Paediatr 1997;17:39–44.
- Saper RB, Kales SN, Paquin J, et al. Heavy metal content of Ayurvedic herbal medicine products. JAMA 2004;292:2868–73.
- World Health Organization. WHO traditional medicine strategy 2002–2005. Geneva, Switzerland: World Health Organization; 2002. Available at http://apps.who.int/medicinedocs/pdf/s2297e/s2297e.pdf. Accessed August 20, 2012.
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Report 2008(12)
- Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA 2008;300:915–23.
* Information available at http://www.fda.gov/forconsumers/consumerupdates/ucm269384.htm.
† Information available at http://www.fda.gov/downloads/forconsumers/consumerupdates/ucm050819.pdf.
§ Available at http://www.fda.gov/safety/medwatch/howtoreport/default.htm.
What is already known on this topic?
Foreign-born populations, including pregnant women, might have increased risk for lead poisoning because of their use of foreign medications or dietary supplements containing high levels of lead. Numerous cases of heavy metal poisonings associated with the use of health products made or brought from abroad have been documented.
What is added by this report?
During 2011–2012, six pregnant women with elevated blood lead levels were identified in New York City as a result of required prenatal screening. All six were foreign-born users of oral Ayurvedic medications made in India. The products used contained up to 2.4% lead, and several contained mercury and arsenic, which also can have adverse health effects.
What are the implications for public health practice?
Products containing lead and other heavy metals are available to consumers through travel abroad or other channels in which regulation is limited or unenforceable. When lead poisoning is suspected, public health workers and health-care providers should consider as potential risk factors the use of foreign medications, dietary supplements, or traditional remedies, especially among foreign-born persons and, importantly, among pregnant women. Health-care providers should advise patients to stop using foreign products that might contain heavy metals and consider testing patients for exposure to lead or other heavy metals if use is reported.
|
TABLE. (Continued) Ayurvedic medications and other health remedies that have been identified with high heavy metal content — New York City Department of Health and Mental Hygiene, 2004–2012 |
||||||
|---|---|---|---|---|---|---|
|
Product |
Manufacturer |
Country where manufactured |
Country where purchased |
Usage reported or labeled |
Heavy metal content |
Rasa shastra* |
|
Mahashakti Rasayan |
Vyas Pharmaceuticals |
India |
India |
Weakness |
9,400 ppm Pb 70,000 ppm Hg 1,700 ppm As |
Yes |
|
Mahayogaraj Guggulu (enriched with silver) |
Baidyanath |
India |
United States |
Rheumatic pain |
47,000 ppm Pb 4,800 ppm Hg 4,300 ppm As |
Yes |
|
Ovarin§ |
Ban Labs Ltd. |
India |
India |
Facilitate ovulation Reproductive health |
24,000 ppm Pb 70,000 ppm Hg 1,000 ppm As |
Yes |
|
Pigmento§ |
Charak Pharma Pvt. Ltd. |
India |
India |
Improve skin complexion |
29,000 ppm Hg 27 ppm As 7.3 ppm Pb |
Yes |
|
Pregnita§ |
Ajmera Pharmaceuticals |
India |
India |
Pregnancy-related nausea and vomiting |
12,000 ppm Pb |
Yes |
|
Sorin |
Research Drugs & Pharmaceuticals |
India |
India |
Eczema |
46,707 ppm Pb |
Yes |
|
Tierra Santa |
Unknown |
Mexico |
United States |
Cleanse stomach for pregnancy |
13 ppm Pb 11 ppm As |
NA |
|
Vasant Kusumakar Ras (with Gold and Pearl) |
Dabur |
India |
India |
Diabetes and weakness |
29 ppm Pb 31,000 ppm Hg |
Yes |
|
Vatvidhwansan Ras§ |
Baidyanath |
India |
United States |
Skin problems |
20,000 ppm Pb 15,000 ppm Hg 130 ppm As |
Yes |
|
Vita Breath |
American Herbal Laboratories |
United States |
United States |
Incontinence and to test the "strength of meridians" |
1,100 ppm Pb |
NA |
|
Abbreviations: Pb = lead; Hg = mercury; As = arsenic; NA = not applicable (product is not Ayurvedic). * Rasa shastra is the ancient practice of deliberately combining herbs with "bhasmas" (elaborately prepared metal, mineral, and gem compounds perceived to be safe) to enhance therapeutic benefit. Ayurvedic medications are divided into two major categories: herbal only and rasa shastra. Source: Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA 2008;300:915–23. † Lot no. 010705, expiration date: July 2010. § The 10 products associated with the six cases described in this report. ¶ Lot no. 040804, expiration date: August 2009; lot no. 100105, expiration date: January 2010; lot no. 110604, expiration date: June 2009. |
||||||
FIGURE. Two of 10 Ayurvedic medications associated with lead poisoning in six pregnant women — New York City Department of Health and Mental Hygiene, 2011–2012
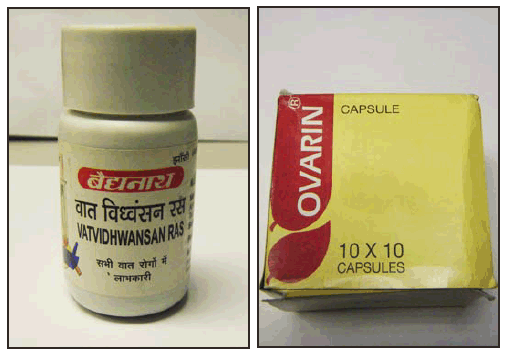
Photos / New York City Department of Health and Mental Hygiene
Alternate Text: The figure above shows two of 10 Ayurvedic medications that have been associated with lead poisoning in six pregnant women during 2011-2012, according to the New York City Department of Health and Mental Hygiene. Patient 2 reported purchasing Vatvidhwansan Ras, which contained 2% lead in New York City. Patient 4 obtained Ovarian, which was found to have as much as 1.2% lead, in India.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


