Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Multistate Outbreak of Fungal Infection Associated with Injection of Methylprednisolone Acetate Solution from a Single Compounding Pharmacy — United States, 2012
On October 12, 2012, this report was posted as an MMWR Early Release on the MMWR website (http://www.cdc.gov/mmwr).
On September 18, 2012, the Tennessee Department of Health was alerted by a clinician regarding a patient with culture-confirmed Aspergillus fumigatus meningitis diagnosed 46 days after epidural steroid injection at a Tennessee ambulatory surgical center. By September 27, the initial investigation, carried out by the Tennessee Department of Health in collaboration with CDC and the North Carolina Department of Health and Human Services, had identified an additional eight patients with clinically diagnosed, culture-negative meningitis: seven in Tennessee and one in North Carolina. All nine patients had received epidural steroid injection with preservative-free methylprednisolone acetate solution (MPA), compounded at New England Compounding Center (NECC) in Framingham, Massachusetts. All nine patients had received one or more injections from three lots of MPA (lot numbers 05212012@68; 06292012@26; and 08102012@51). As of October 10, a multistate investigation led by CDC in collaboration with state and local health departments and the Food and Drug Administration (FDA) had identified 137 cases and 12 deaths associated with this outbreak in 10 states. Active case-finding efforts and extensive investigation into medications and medication lot numbers received by patients have confirmed that, as of October 10, no cases were associated with other lots of MPA, nor were any associated with other NECC products. This report describes the ongoing investigation by CDC and state and local health departments, and includes important recommendations for physicians and patients.
NECC was informed of the ongoing investigation on September 25 and provided invoice information indicating that approximately 17,500 vials of MPA (80 mg/ml) from these lots were packaged in 1ml, 2ml, and 5ml vials and distributed to 75 facilities in 23 states. These lots of MPA were used to treat both peripheral joint and back pain. On September 26, NECC voluntarily recalled the three lots of MPA, followed by an expanded voluntary recall of all lots of MPA and all lots of sterile products intended for intrathecal injection on October 3. This was followed by a voluntary recall of all remaining products on October 6.
Some patients received multiple injections with the three lots of MPA, and some vials were unused. As of October 10, state and local health departments had identified almost 14,000 persons potentially exposed to medications from at least one of these lots. Active notification of exposed persons was initiated by state health departments and CDC on September 25. Passive case finding was conducted by widely disseminated notices of the potential contamination of the three MPA lots via Epi-X (a CDC electronic public health notification system), through professional societies and listservs, and the news media. As of October 10, state health departments had reported that approximately 90% of patients exposed to medication from one of the three lots of MPA recalled on September 26 had been contacted at least once, by telephone, voicemail, home visit, or registered mail.
As of October 10, four categories of cases in patients who received an injection with MPA produced by NECC had been identified: 1) fungal meningitis or nonbacterial and nonviral meningitis of subacute onset following epidural injection on or after May 21; 2) basilar stroke following epidural injection on or after May 21, in a person from whom no cerebrospinal fluid (CSF) specimen was obtained; 3) spinal osteomyelitis or epidural abscess at the site of injection following epidural or sacroiliac injection on or after May 21; 4) septic arthritis or osteomyelitis of a peripheral joint (e.g., knee) diagnosed following injection of that joint on or after May 21. Clinical meningitis was defined as having one or more symptoms (e.g., headache, fever, stiff neck, or photophobia) and CSF pleocytosis (more than five white blood cells per µL, adjusting for presence of red blood cells), regardless of CSF protein and glucose levels. Clinically diagnosed septic arthritis was defined as new or worsening pain with presence of effusion or new or worsening effusion.
As of October 10, 137 patients in 10 states had been identified who met one or more of the four definitions, all of whom underwent injection with one or more of the three lots of MPA from NECC. No cases associated with other lots of MPA, or other NECC products, had been identified. Twelve (9%) of the 137 patients died. Preliminary data are available on 70 (51%) patients. Of these, 64 (91%) have meningitis (case definition 1). Of the six remaining patients, two (3%) have stroke without lumbar puncture (definition 2), and two (3%) have an epidural abscess or osteomyelitis (definition 3). Two (3%) patients met more than one case definition (definitions 1 and 3).
Median age of the 70 patients is 68 years (range: 23–91 years); 48 (69%) are female. At presentation, 57 (81%) had headache, 24 (34%) had fever, 21 (30%) had nausea, and seven (10%) had photophobia (Table). Atypical neurologic symptoms were observed in a minority of patients; subtle gait disturbances were seen in three (4%), and a history of falls was described in eight (11%). Meningeal signs, including nuchal rigidity, Kernig's sign, or Brudzinski's sign, were uncommon, occurring in 10 (14%) patients (Table). Stroke, either as a presenting sign, or as a complication of infection, occurred in 12 (17%) (Table).
Median CSF white blood cell count was 1,299/µL (range: 13–15,400) with a neutrophilic predominance; median CSF glucose was 42 mg/dL (range: 11–121), and median protein was 129 mg/dL (range: 45–588). As of October 10, evidence of a fungal infection had been found in 26 (37%) patients by culture, histopathology, or polymerase chain reaction. The fungal species had been identified in 14 patients; Exserohilum spp was identified in 13, and Aspergillus fumigatus was identified in one patient (Table). Further specimen evaluation is ongoing at CDC and state public health and local laboratories.
For the 61 patients with symptom onset date available, the earliest date was August 18 (Figure). For the 48 patients with both injection date and symptom onset date available for analysis, the median time from last steroid injection to onset of symptoms was 15 days (range: 1–42). A total of 25 of the 48 patients received a single steroid injection; the median time from injection to onset of symptoms for these patients was 16 days (range: 4–42).
Reported by
Marion Kainer, MD, Andrew D. Wiese, MPH, Tennessee Dept of Health. Kaitlin Benedict, MPH, Chris Braden, MD, Mary Brandt, PhD, Julie Harris, PhD, Benjamin J. Park, MD, Div of Foodborne, Waterborne, and Environmental Diseases; Alice Guh, MD, John Jernigan, MD, Melissa Schaefer, MD, J. Todd Weber, MD, Matt Wise, PhD, Div of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Diseases; Rachel M. Smith, MD, Duc Nguyen, MD, EIS officers, CDC. Corresponding contributor: Rachel M. Smith MD, vih9@cdc.gov, 404-639-7738.
Editorial Note
Meningitis and parameningeal infections are extremely rare complications of epidural injection, with few cases reported (1–3). Most often these infections are bacterial; rarely is a postepidural injection meningitis case caused by fungi, and when present, fungal infection is often diagnosed only after a patient fails to improve on antibiotic therapy. Diagnosis of fungal meningitis, particularly in cases caused by molds, is difficult because traditional diagnostic methods such as culture have a low yield (4–6). Molecular methods such as polymerase chain reaction have been useful for detection in some cases, but currently remain in use only as research tools.
The clinical presentation of fungal meningitis is often indolent, with few patients displaying the classic meningeal signs of bacterial meningitis. Early in this outbreak, many patients with meningitis had only a few mild symptoms, but had CSF pleocytosis. Additionally, some of these patients either presented with, or later developed, a stroke in the posterior circulation (which supplies the cerebellum, midbrain, and brainstem), a finding described in one prior case series of fungal meningitis (6). Clinicians should be aware of the atypical presentation of meningitis in this outbreak, and should consider performing lumbar puncture if patients have mild symptoms and have received a steroid injection originating from one of the three implicated lots of MPA. Early identification and treatment of patients with fungal infections might reduce the risk for serious complications, such as stroke or death. It is possible that the lower case-fatality rate reported here compared with other case series might have resulted from intensive patient notification and earlier diagnosis and therapy; further investigation is needed.
Close follow-up of these patients is needed to understand important clinical questions, such as the optimal medications, dosage, and duration of treatment. To provide guidance on these important clinical issues, CDC, in consultation with experts in the diagnosis and treatment of fungal infections, has drafted interim treatment guidelines for infections associated with this outbreak. Current recommendations for treatment of central nervous system and parameningeal infections include consultation with an infectious disease physician and initiation of empiric antifungal therapy with high dose voriconazole and liposomal amphotericin B. Treatment duration is likely to be prolonged, on the order of months, and will need to be tailored to individual patients. Routine use of adjuvant steroids or intrathecal amphotericin B in treatment and postexposure prophylaxis or screening of asymptomatic persons by lumbar puncture currently are not recommended. These recommendations are subject to change as more information becomes available.
As of October 6, all products manufactured since January 1, 2012, have been recalled by NECC and should not be used. The FDA and Massachusetts Board of Registration in Pharmacy investigation into the NECC facility is ongoing and includes microbiologic testing of unopened vials of the three lots of MPA as well as additional NECC products. If not already completed, providers should contact all patients exposed to any of the three lots of MPA recalled on September 26 to inquire about symptoms. Patients who received epidural injection with medication from any of the three lots of MPA and who have symptoms of meningitis or posterior circulation stroke should be referred for diagnostic lumbar puncture, if not contraindicated. Patients with signs or symptoms of parameningeal infection or peripheral joint infection (e.g., increasing pain, redness, or swelling at the injection site) should be referred for diagnostic evaluation, which might include aspiration of fluid collections or joint aspiration. Although available preliminary data demonstrate incubation periods ranging from 4 to 42 days, the maximum incubation period for this infection is not known; therefore, asymptomatic but exposed patients should remain vigilant for symptoms and seek medical attention should symptoms develop. More guidance for patients and clinicians, including interim treatment guidelines, is available at http://www.cdc.gov/hai/outbreaks/meningitis.html.
Acknowledgments
Multistate Meningitis Outbreak Response Team: Doug Kernodle, MD, April C. Pettit, MD, Kathy Wilkerson, and the Fungal Infection Team, Vanderbilt Univ Medical Center. Carol A. Rauch, MD, Jonathan E. Schmitz, MD, Vanderbilt Univ School of Medicine. Megan Casey, MPH, Allegheny County Health Dept. Srihari Seshadri, MBBS, Barren River District Health Dept. Jon Rosenberg, MD, California Dept of Public Health. Stephanie R. Black, MD, Alicia Siston, PhD, Chicago Dept of Public Health. Michael O. Vernon, DrPh, Cook County Dept of Public Health. Wendy Chung MD, Dallas County Health and Human Svcs. Carina Blackmore, DVM, Tricia Foster, MPH, Florida Dept of Health. Christine Hahn, MD, Kathy Turner, PhD, Idaho Div of Public Health. Judith Conway, Kate Kelly-Shannon, Matthew Roberts, MPH, Kenneth Soyemi, MD, Illinois Dept of Public Health. Pamela Pontones, MA, Joan Duwve, MD, Indiana State Dept of Health. Kraig Humbaugh, MD, Kentucky Dept for Public Health. Clara Tyson, MSN, Los Angeles County Dept of Public Health. Amy Reilly, Meghan Gumke, Nathan Grossman, MD, Marion County Health Dept. Madeleine Biondolillo, MD, James D. Coffey, Jim Collins, MPH, Joseph Coyle, MPH, Kevin Cranston, Alfred DeMaria, MD, Jean Pontikas, Iyah Romm, Massachusetts Dept of Public Health. David Blythe, MD, Ruth Thompson, Lucy Wilson, Maryland Dept of Health and Mental Hygiene. Craig McMilllan, MD, Mendocino County Dept of Public Health. Jay Fiedler, MS, Jennie Finks, DVM, Shannon Johnson, MPH, Michigan Dept of Community Health. Kathryn Como-Sabetti, MPH, Richard Danila, PhD, Aaron DeVries, MD, Jane Harper, Ruth Lynfield, MD, Minnesota Dept of Health. Ihsan Azzam, MD, PhD, Patricia Rowley, MPH, Nevada Dept of Health and Human Svcs. Sharon Alroy-Preis, MD, New Hampshire Dept of Health and Human Svcs. Barbara Carothers, Barbara Montana, MD, Christina Tan, MD, Laura Taylor, PhD, New Jersey Dept of Health. Jean-Marie Maillard, MD, Zack Moore, MD, North Carolina Dept of Health and Human Svcs. Mary DiOrio, MD, Ohio Dept of Health. Mary Powell, MPH, Pennyrile District Health Dept. Virginia Dato, MD, Elizabeth Hunt, MPH, Erica E. Smith, MD, Lauren Torso, MPH, Pennsylvania Dept of Health. Caroline Banis, Dan Drociuk, MD, South Carolina Dept of Health and Environmental Control. Brynn E. Berger, MPH, Meredith L. Kanago, MSPH, Jea Young Min, PharmD, David Reagan, MD, PhD, Brenda Rue, Jennifer Ward, MS, Anita Kurian, DrPh, Tarrant County Public Health. Neil Pascoe, Texas Dept of State Health Svcs. Karen Haught, MD, Tulare County Dept of Public Health. Katie Kurkjian, DVM, Margaret Tipple, MD, David Trump, MD, Virginia Dept of Health. Danae Bixler, MD, Carrie A. Thomas, PhD, West Virginia Bur for Public Health. Susann E. Ahrabi-Fard, MS, Richard T. Heffernan, MPH, James J. Kazmierczak, DVM, Wisconsin Div of Public Health. Aaron Fleischauer, PhD, James Lando, MD, Jevon McFadden, MD, Doug Thoroughman, PhD, Tom Torok, MD, Career Epidemiology Field Officers, Office of Public Health Preparedness and Response; Tom Chiller, MD, Angela Cleveland, MPH, Martha Iwamoto, MD, Ana Litvenseva, PhD, Shawn Lockhart, PhD, Agam Rao, MD, Monika Roy, MD, Jonathan Yoder, MPH, Div of Foodborne, Waterborne, and Environmental Diseases, Shelley Magill, MD, PhD, Div of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Diseases; Anne Purfield, PhD, Jane Baumblatt, MD, Amanda Beaudoin, PhD, Jennifer Espiritu, MD, Stephanie Griese, MD, Michael Gronostaj, MD, Christina Mikosz, MD, Maria Said, MD, Alice Shumate, MD, EIS officers, CDC.
References
- Cooper AB, Sharpe MD. Bacterial meningitis and cauda equina syndrome after epidural steroid injections. Can J Anaesth 1996;43(5 pt 1):471–4.
- Snarr J. Risk, benefits and complications of epidural steroid injections: a case report. AANA J 2007;75:183–8.
- Kolbe AB, McKinney AM, Kendi AT, Misselt D. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol 2007;48:687–9.
- Verweij PE, Brinkman K, Kremer HP, Kullberg BJ, Meis JF. Aspergillus meningitis: diagnosis by non-culture-based microbiological methods and management. J Clin Microbiol 1999;37:1186–9.
- Viscoli C, Machetti M, Gazzola P, et al. Aspergillus galactomannan antigen in the cerebrospinal fluid of bone marrow transplant recipients with probable cerebral aspergillosis. J Clin Microbiol 2002;40:1496–9.
- Rodrigo N, Perera KN, Ranwala R, Jayasinghe S, Warnakulasuriya A, Hapuarachchi S. Aspergillus meningitis following spinal anaesthesia for caesarean section in Colombo, Sri Lanka. Int J Obstet Anesth 2007;16:256–60.
FIGURE. Number of cases (n = 61) of fungal infection with known date of symptom onset following epidural steroid injection of methylprednisolone acetate from New England Compounding Center, by date of symptom onset — United States, 2012
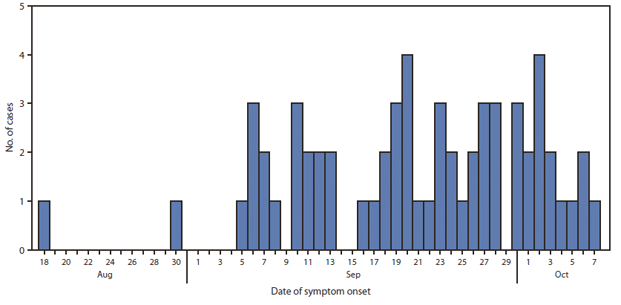
Alternate text: The Figure above shows the number of cases of fungal infection with known date of symptom onset, following epidural steroid injection of methylprednisolone acetate from New England Compounding Center, by date of symptom onset - United States, 2012
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


