Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Evaluation of 11 Commercially Available Rapid Influenza Diagnostic Tests — United States, 2011–2012
Accurate diagnosis of influenza is critical for clinical management, infection control, and public health actions to minimize the burden of disease. Commercially available rapid influenza diagnostic tests (RIDTs) that detect the influenza virus nucleoprotein (NP) antigen are widely used in clinical practice for diagnosing influenza because they are simple to use and provide results within 15 minutes; however, there has not been a recent comprehensive analytical evaluation of available RIDTs using a standard method with a panel of representative seasonal influenza viruses. This report describes an evaluation of 11 Food and Drug Administration (FDA)–cleared RIDTs using 23 recently circulating influenza viruses under identical conditions in a laboratory setting to assess analytical performance. Most RIDTs detected viral antigens in samples with the highest influenza virus concentrations, but detection varied by virus type and subtype at lower concentrations. Clinicians should be aware of the variability of RIDTs when interpreting negative results and should collect test samples using methods that can maximize the concentration of virus antigen in the sample, such as collecting adequate specimens using appropriate methods in the first 24–72 hours after illness onset. The study design described in this report can be used to evaluate the performance of RIDTs available in the United States now and in the future.
As part of a collaboration between CDC, the Biological Advanced Research and Development Authority, and the Medical College of Wisconsin (MCW), CDC provided 16 influenza A and seven influenza B viruses to MCW to evaluate RIDTs commercially available during the 2011–12 influenza season (Table). Stock viruses were representative of viruses circulating in the United States since 2006 and were characterized by their 50% egg infectious dose (EID50/mL, a measure of virus infectivity). In addition, the concentration of influenza virus NP antigen (the antigen detected by RIDTs) was measured as µg/mL using isotope dilution tandem mass spectrometry (1). EID50/mL values were at least as high as those reported in human clinical specimens (2–4). MCW prepared swab samples or mock nasal wash specimens from several dilutions of each virus in saline. For nine of 11 RIDTs, 50 µL of virus dilution was applied to swabs provided in the test kit or swabs described in the manufacturer's instructions for use. Two RIDTs (both manufactured by SA Scientific) require use of nasal wash specimens. Therefore, for the SA Scientific tests, 50 µL from each virus dilution first was added to saline. All samples, either prepared swabs or liquid, were added to RIDTs and incubated, with results interpreted as described in the instructions for use. Three separate tests were performed for each combination of virus and RIDT.
The numbers of RIDTs that were positive (defined as at least two positive results of the three tests performed) at each dilution for each of the 23 influenza viruses were compared (Table). RIDTs overall had fewer positive results with viruses that had the lowest stock NP concentrations (<2 µg/mL). Each influenza virus had variable levels of positivity with RIDTs, suggesting that several viruses of each type and subtype should be evaluated with each RIDT on a regular basis. NP levels of influenza B virus stocks generally were higher, and the first two dilutions were detected more uniformly than for influenza A viruses. No significant performance differences were noted for B/Victoria or B/Yamagata lineages of influenza B viruses.
The numbers of positive test results for each of the 11 RIDTs by influenza virus type and influenza A subgroup were compared (Figure). One RIDT (SAS FluAlert Influenza A [SA Scientific]) did not uniformly detect influenza A (H1N1)pdm09 (pH1N1) viruses or other influenza A viruses at high concentrations. Four RIDTs detected the majority of influenza B viruses in third dilution samples, whereas only one RIDT (BD Directigen EZ Flu A+B [Becton, Dickinson and Co.]) detected at least 50% of all influenza A viruses in third dilution samples.
Reported by
Eric Beck, PhD, Jiang Fan, MD, Kelly Hendrickson, MD, Swati Kumar, MD, Midwest Respiratory Virus Program, Dept of Pediatrics, Medical College of Wisconsin. Roxanne Shively, MS, William Kramp, PhD, Biomedical Advanced Research and Development Authority, US Dept of Health and Human Svcs. Julie Villanueva, PhD, Daniel Jernigan, MD, Alexander Klimov, PhD, Li-Mei Chen, Ruben Donis, PhD, Influenza Div, National Center for Immunization and Respiratory Diseases; Tracie Williams, James Pirkle, MD, PhD, John Barr, PhD, Div of Laboratory Science, National Center of Environmental Health, CDC. Corresponding contributor: Roxanne Shively, roxanne.shively@hhs.gov, 202-260-1651.
Editorial Note
Before the emergence of influenza A (H1N1)pdm09 viruses in 2009, published reports showed variable performance of RIDTs, with reported sensitivities ranging from 27% to 61% when compared with real-time reverse transcription–polymerase chain reaction (PCR) testing (5). During the A (H1N1)pdm09 pandemic, clinicians, researchers, and regulators questioned whether RIDTs could detect the newly emerging virus. CDC reported that 1) sensitivities of three commonly used RIDTs ranged from 40% to 69% with influenza A (H1N1)pdm09 archived clinical specimens compared with CDC's A (H1N1)pdm09 PCR assay and 2) higher virus loads led to a greater likelihood of a positive result (6). These findings prompted recommendations for clinicians to use caution when interpreting results of RIDTs. Recently, the performances of selected RIDTs for detecting the influenza A (H3N2) variant virus were evaluated and were found to vary considerably (7).
Previous reports of RIDT performance often used different volumes or amounts of virus propagated under different conditions and did not evaluate the majority of commercially available, FDA-cleared RIDTs. For the evaluation described in this report, efforts were made to 1) use identical viral concentrations for each kit tested, 2) use all 11 commercially available, FDA-cleared RIDTs for the 2010–11 influenza season, and 3) use a diverse collection of 23 more recent influenza viruses to allow for a more finely detailed characterization of test performance. The analytical sensitivity of the evaluation varied across test kits as well as with different influenza viruses, indicating that test performance for some RIDTs drops significantly with decreasing virus concentration.
The findings in this report further emphasize the importance of collecting respiratory specimens when the amount of influenza virus is at its peak (within 24–72 hours of symptom onset). The high virus concentrations at which the evaluated FDA-cleared RIDTs detected recent circulating viruses might exceed levels expected in clinical specimens, even those collected at the peak of virus load in the specimen (2–4). Although all RIDTs were able to detect virus at the highest virus concentrations, some were unable to detect certain viruses at any subsequent dilution. Manufacturers use different antibodies in their RIDTs to capture NP antigen, and this difference in antibody selection might account for some of the variation in performance. Periodic evaluation of RIDT performance in detecting current or recently circulating influenza viruses might identify needed updates in antibodies used in commercial RIDTs. In addition, given the narrow range of virus concentrations that can be detected by the majority of RIDTs, clinicians should follow best practices for specimen collection and timing to maximize the number of influenza viruses per specimen and improve the clinical utility of the test.
These findings do not reflect the RIDTs' performance in clinical settings. Ideally, RIDT performance should be evaluated using respiratory specimens from patients with influenza-like illnesses; however, performing a study to evaluate the performances of 11 RIDTs using specimens collected in a standard manner from enough patients with influenza-like illness to include 23 circulating influenza viruses presents a tremendous challenge. The methods described in this report avoid the variability in the quality and virus concentration of specimens inherent in clinical studies. This evaluation also provides a baseline for assessing analytical variability with RIDTs over time as human seasonal influenza viruses evolve, and for rapidly determining RIDT performance as novel influenza viruses emerge.
Clinicians and laboratorians should be aware of the limitations of RIDTs. Performance reported in analytical studies depends on the characteristics of selected viruses and their growth characteristics as well as the affinity of antibodies used in RIDTs. These findings highlight the need for clinicians and laboratorians to use RIDTs cautiously for diagnostic, treatment, and infection control decisions in clinical settings. Because of variability in RIDT performance, especially at lower viral concentrations, negative RIDT test results might not exclude influenza virus infection in patients with signs and symptoms suggestive of influenza. Therefore, antiviral treatment, if indicated, should not be withheld from patients with suspected influenza because they have a negative RIDT test result (8). Clinicians and laboratorians can take measures to improve detection of influenza, such as 1) collecting specimens early in the course of illness, 2) ensuring that the appropriate type and highest quality of respiratory specimen is collected, and 3) using the current local prevalence of influenza activity to raise or lower the suspicion of influenza and to assess the benefit of testing (9).
The use of RIDTs in clinical management and public health practice can be improved by continually updating guidance, educating clinicians on best practices, and enhancing test design for better performance. To this end, the Joint Commission, in its role to improve clinical practice, is offering two Internet-based courses, including a continuing education course, on Strategies for Improving Rapid Influenza Testing in Ambulatory Settings. Course descriptions and registration information are available at http://www.jointcommission.org/siras.aspx, as are a number of links to online resources on the use and interpretation of RIDTs. In addition, a dedicated YouTube channel for Strategies for Improving Rapid Influenza Testing in Ambulatory Settings features several instructional videos on the subject (available at http://www.youtube.com/playlist?list=PLNQfL_CJ36fK08KEPjxu1ZKJn7GuFtn-N&feature=plcp).
Acknowledgments
Michael Bose, Sagarika Tiwari, Shamim Khaja, Hong Mei, Michael Ulatowski, Medical College of Wisconsin. Supporting staff, Biological Advanced Research and Development Authority, US Dept of Health and Human Svcs. Paul Gargiullo, Stephen Lindstrom, Christine Warnes, and the Influenza Reagent Resource Program, Influenza Div, National Center for Immunization and Respiratory Diseases, CDC.
References
- Williams TL, Luna L, Guo Z, et al. Quantification of influenza virus hemagglutinins in complex mixtures using isotope dilution tandem mass spectrometry. Vaccine 2008;26:2510–20.
- Calfee DP, Peng AW, Cass LM, Lobo M, Hayden FG. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother 1999;43:1616–20.
- Döller G, Schuy W, Tjhen KY, Stekeler B, Gerth HJ. Direct detection of influenza virus antigen in nasopharyngeal specimens by direct enzyme immunoassay in comparison with quantitating virus shedding. J Clin Microbiol 1992;30:866–9.
- Kaiser L, Briones MS, Hayden FG. Performance of virus isolation and Directigen Flu A to detect influenza A virus in experimental human infection. J Clin Virol 1999;14:191–7.
- Uyeki TM, Prasad R, Vukotich C, et al. Low sensitivity of rapid diagnostic test for influenza. Clin Infect Dis 2009;48:e89.
- CDC. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus—United States, 2009. MMWR 2009;58:826–9.
- Balish A, Garten R, Klimov A, Villanueva J. Analytical detection of influenza A(H3N2)v and other A variant viruses from the USA by rapid influenza diagnostic tests. Influenza Other Respi Viruses 2012;September 18 [Epub ahead of print].
- CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-1).
- CDC. Guidance for clinicians on the use of rapid influenza diagnostic tests for the 2010–2011 influenza season. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm. Accessed October 23, 2012.
What is already known on this topic?
Accurate diagnosis of influenza is critical for clinical management, infection control, and public health actions. Rapid influenza diagnostic tests (RIDTs) are widely used in clinical practice, but their abilities to detect a range of influenza viruses in recent circulation have not been evaluated comprehensively.
What is added by this report?
Eleven Food and Drug Administration–cleared RIDTs were evaluated using a panel of 23 recently circulating influenza viruses. Most tests detected viral antigen in samples at the highest concentrations, but detection varied by test and viral type and subtype at lower concentrations.
What are the implications for public health practice?
Clinicians should be aware of the variability of RIDTs when interpreting negative results and should collect test samples using methods that can maximize the concentration of virus antigen in the sample by collecting specimens with appropriate methods within 24–72 hours after illness onset. The use of these tests for clinical management and public health practice can be improved by continually updating guidance, educating clinicians on best practices, and enhancing test design for better performance. The study design described in this report can be used for future evaluations of the sensitivity and performance of rapid influenza tests available in the United States.
FIGURE. Number of positive samples in each dilution and percentage of positive samples in each virus group, by RIDT kit — United States, 2012
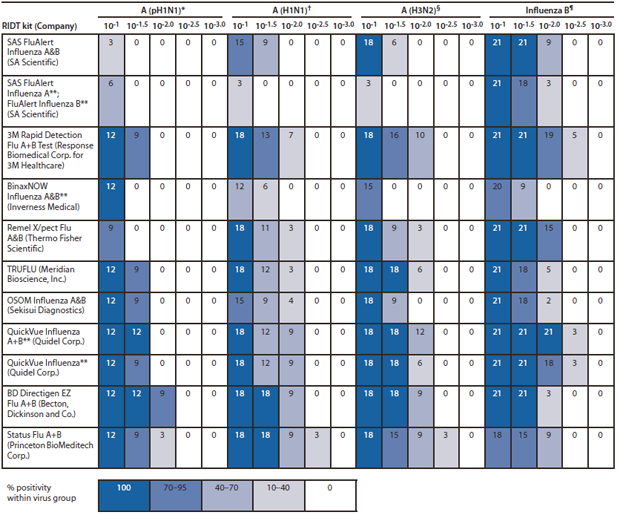
Abbreviation: RIDT = rapid influenza diagnostic test.
* Four influenza A (H1N1) 2009 pandemic (pH1N1) viruses with three samples at each dilution (12 possible positive samples for each dilution).
† Six pre-pandemic "seasonal" influenza A (H1N1) viruses with three samples at each dilution (18 possible positive samples for each dilution).
§ Six influenza A (H3N2) viruses with three samples at each dilution (18 possible positive samples for each dilution).
¶ Seven influenza B viruses with 3 samples at each dilution (21 possible posiive samples for each dilution).
** CLIA-waived (i.e., exempt from all regulatory procedures typically required under Clinical Laboratory Improvement Amendments).
Alternate Text: The figure above shows the number of positive samples in each dilution and percentage of positive samples in each virus group for each of the 11 rapid influenza diagnostic tests (RIDT) kits commercially available in the United States in 2012 and cleared by the Food and Drug Administration. The numbers of positive test results for each of the RIDTs by influenza virus type and influenza A subgroup were compared. One RIDT (SAS FluAlert Influenza A) did not uni¬formly detect influenza A (H1N1)pdm09 (pH1N1) viruses or other influenza A viruses at high concentrations. Four RIDTs detected the majority of influenza B viruses in third dilution samples, whereas only one RIDT (BD Directigen EZ Flu A+B) detected at least 50% of all influenza A viruses in third dilution samples.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


