Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Cervical Cancer Screening Among Women Aged 18–30 Years — United States, 2000–2010
Screening women for cervical cancer can save lives. However, among young women, cervical cancer is relatively rare (1,2), and too-frequent screening can lead to high costs and adverse events associated with overtreatment (3). Before 2012, cervical cancer screening guidelines of the American College of Obstetricians and Gynecologists (ACOG), American Cancer Society (ACS), and U.S. Preventive Services Task Force (USPSTF) differed on age to start and how often to get screened for cervical cancer. (4). In 2012, however, all three organizations recommended that 1) screening by Papanicolau (Pap) test should not be used for women aged <21 years, regardless of initiation of sexual activity, and 2) a screening interval of 3 years should be maintained for women aged 21–30 years. ACS and ACOG explicitly recommend against yearly screening (5–7). To assess trends in Pap testing before the new guidelines were introduced, CDC analyzed 2000–2010 data from the Behavioral Risk Factor Surveillance System (BRFSS) for women aged 18–30 years. CDC found that, among women aged 18–21 years, the percentage reporting never having been screened increased from 26.3% in 2000 to 47.5% in 2010, and the proportion reporting having had a Pap test in the past 12 months decreased from 65.0% to 41.5%. Among those aged 22–30 years, the proportion reporting having had a Pap test within the preceding 12 months decreased from 78.1% to 67.0%. These findings showed that Pap testing practices for young women have been moving toward the latest guidelines. However, the data also showed a concerning trend: among women aged 22–30 years, who should be screened every 3 years, the proportion who reported never having had a Pap test increased from 6.6% to 9.0%. More effort is needed to promote acceptance of the latest evidence-based recommendations so that all women receive the maximal benefits of cervical cancer screening.
BRFSS is a state-based, random-digit–dialed telephone survey of the noninstitutionalized, U.S. civilian adult population aged ≥18 years. During 2000–2010, women respondents were asked, "Have you ever had a Pap test?" Those answering "yes" were asked about the timing of their last test. Pap test status was categorized into four mutually exclusive groups (never, within 12 months, within 13–24 months, and within 25–36 months). Women who reported having had their last Pap test >36 months ago (<5%) were not categorized. Survey response rates ranged from 46.0% to 55.7% over the 10-year study period.
Data were collected during 2000–2010 from 125,297 women aged 18–30 years who lived in the 50 states and the District of Columbia. Pap test status was analyzed by age group, race/ethnicity, U.S. Census region,* and health-care coverage status. Unadjusted logistic regression models were used to test for statistical differences in Pap testing behaviors over the 10-year period, with year treated as a categorical variable. Percentages and 95% confidence intervals were calculated; differences in percentages were considered statistically significant at p≥0.05. All analyses were performed using statistical software to account for the complex sampling design.
Among women aged 18–21 years, an increase from 26.3% in 2000 to 47.5% in 2010 was observed in the percentage reporting never having been tested (Table 1), and a decrease from 65.0% in 2000 to 41.5% in 2010 was observed in the percentage reporting a Pap test within the preceding 12 months (Figure). Among women in this age group, an increase in reporting never having been tested was observed in all racial/ethnic categories (Table 1).
Among women aged 22–30 years, a decrease from 78.1% in 2000 to 67.0% in 2010 was observed in the percentage reporting a Pap test within the preceding 12 months (Table 2). From 2000 to 2010, increases were observed in the percentages of women aged 22–30 years who reported having had a Pap test within the preceding 13–24 (9.8% to 15.3%) and 25–36 months (2.6% to 4.5%) (Figure). Among women in this age group, increases in reported Pap tests within the preceding 13–36 months were observed in all racial/ethnic categories (Table 2).
Whereas more women who did not need screening reported not being screened, an increase also was observed in the number of women who did need screening but reported not being screened. Among women aged 18–21 years, the percentage who reported never having had a Pap test increased from 26.3 to 47.5%, whereas, among women aged 22–30 years, the percentage who reported never having had a Pap test increased from 6.6% in 2000 to 9.0% in 2010.
Reported by
Mona Saraiya, MD, Jessica King, MPH, Trevor Thompson, Meg Watson, MPH, Umed Ajani, MBBS, Jun Li, MD, PhD, Div of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion; Keisha A. Houston, DrPH, EIS Officer, CDC. Corresponding contributor: Keisha A. Houston, kahouston@cdc.gov, 770-488-3096.
Editorial Note
Advances in scientific knowledge have led to recent cervical cancer screening recommendations to begin Pap testing at age 21 years and receive screening every 3 years. Among young women, invasive cervical cancer is rare, with approximately 125 women aged <25 years receiving a cervical cancer diagnosis annually. Most precancerous lesions detected by Pap testing regress, even without treatment (1,2). Previous studies also have shown that frequent testing and overtreatment of women can lead to harm associated with diagnostic procedures (3), including adverse birth outcomes (6,8).
The findings in this report show that increasing numbers of women are being screened at an age and with a frequency consistent with the latest guidelines. However, these data also show that, in 2010, among women aged 22–30 years, 9.0% reported never having had a Pap test. Public health initiatives to increase screening among these women should continue. Interventions designed to increase women's comfort with and knowledge of the rationale behind changes in the screening recommendations might be instrumental in addressing resistance to less frequent screenings (9). However, with more consistent screening guidelines in 2012, a greater number of women aged 18–30 years might be expected to conform with recommendations for when to start and how often to get screened.
The findings in this report are subject to at least three limitations. First, these results might not be representative of all women in the United States because of the low survey response rates and noncoverage of cellular telephone users during the 2000–2010 study period. Second, all information was self-reported and not confirmed by review of medical records. Finally, this survey did not consider whether Pap testing behaviors varied by human papillomavirus (HPV) vaccination status or by timing of sexual initiation.† HPV vaccination is expected to reduce the incidence of cervical cancer substantially. Future national surveys of cervical cancer screening should be able to distinguish between the prevalences of cervical cancer screening in HPV-vaccinated and unvaccinated populations. Surveys also should be able to account for HPV testing, which is recommended with the Pap test as an alternative screening strategy in women aged ≥30 years but explicitly not recommended for screening in women aged <30 years. Both of these newer interventions will affect how to interpret screening rates and intervals.
This report provides baseline measurements for the prevalence of Pap testing among women aged 18–30 years before the 2012 cervical cancer screening guidelines were issued. Early adoption of the 2009 ACOG guidelines recommending that Pap testing begin at age 21 years regardless of sexual history (10) or increased knowledge of the potential harms associated with screening women of childbearing age might explain the increases in women aged 18–21 years reporting never having been screened and women in that age group reporting having an increased interval (>12 months) since their most recent Pap test.
References
- Benard VB, Watson M, Castle P, Saraiya M. Cervical carcinoma rates among young women in the United States. Obstet Gynecol 2012;120:1117–23.
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 2011;103:368–83.
- Moyer V, US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–91.
- Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med 2010;170:977–85.
- CDC. Cervical cancer screening guidelines for average-risk women. Atlanta, GA: US Department of Health and Human Services, CDC; 2012. Available at http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed December 21, 2012.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012;137:516–42.
- American Congress of Obstetricians and Gynecologists. ACOG practice bulletin no. 131: screening for cervical cancer. Obstet Gynecol 2012;120:1222–38.
- Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG 2011;118:1031–41.
- Maclaughlin KL, Angstman KB, Flynn PM, Schmitt JR, Weaver AL, Shuster LT. Predictors of patient comfort and adherence with less frequent cervical cancer screening. Qual Prim Care 2011;19:355–63.
- American Congress of Obstetricians and Gynecologists. ACOG practice bulletin no. 109: screening for cervical cancer. Obstet Gynecol 2009;114:1409–20.
* Northeast: New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont) and Middle Atlantic (New Jersey, New York, Pennsylvania); Midwest: East North Central (Illinois, Indiana, Michigan, Ohio, Wisconsin) and West North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota); South: South Atlantic (Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia), East South Central (Alabama, Kentucky, Mississippi), and West South Central (Arkansas, Louisiana, Oklahoma, Texas); West: Mountain (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming) and Pacific (Alaska, California, Hawaii, Oregon, Washington).
† Women aged >21 years who have not engaged in sexual intercourse might not need a Pap test, depending on circumstances. The decision should be made at the discretion of the women and her physician. Additional information is available at http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf.
What is already known on this topic?
Cervical cancer screening by Papanicolau (Pap) test reduces cancer deaths by detecting precancerous lesions early so that they can be treated before cancer develops. Annual screening beginning as early as age 18 years is no longer recommended. Evidence-based guidelines from the American Cancer Society, American College of Obstetricians and Gynecologist, and U.S. Preventive Services Task Force in 2012 call for screening to begin no earlier than age 21 years and with an interval of 3 years between routine Pap tests for women aged 22–30 years.
What is added by this report?
Analyses of Behavioral Risk Factor Surveillance System data show that Pap test initiation at later ages and longer screening intervals began before the release of the new guidelines. From 2000 to 2010, the proportion of women aged 18–21 years who had never been screened increased from 26.3% to 47.5%, and among women aged 22–30 years, the proportion who reported having a Pap test within the preceding 12 months decreased from 78.1% to 67.0%. These favorable trends are partially counterbalanced by an unfavorable trend; the prevalence of women aged 22–30 years reporting having never been screened increased from 6.6% in 2000 to 9.0% in 2010.
What are the implications for public health practice?
Newer cervical cancer screening guidelines allow screening to focus on women who are at highest risk for cervical cancer. Monitoring screening behaviors of women eligible for screening will be an important step to further reduce disparities in cervical cancer screening.
|
TABLE 2. (Continued) Percentage of women aged 22–30 years who reported never having received a Pap test or having received a Pap test within the preceding 12 months, or within the preceding 13–36 months, by age group, race/ethnicity, U.S. Census region, and health-care coverage status — Behavioral Risk Factor Surveillance System, United States, 2000 and 2010 |
|||||||
|---|---|---|---|---|---|---|---|
|
Characteristic |
2000 |
2010 |
% change 2000 to 2010 |
||||
|
Sample size |
% |
(95% CI) |
Sample size |
% |
(95% CI) |
||
|
Pap test within the preceding 13–36 months† |
|||||||
|
Total |
1,899 |
12.4 |
(11.6–13.3) |
2,758 |
19.8 |
(18.7–20.9) |
59.7 |
|
Age group (yrs) |
|||||||
|
22–24 |
455 |
10.4 |
(9.0–12.0) |
576 |
18.5 |
(16.4–20.8) |
77.9 |
|
25–27 |
618 |
11.6 |
(10.2–13.1) |
908 |
20.9 |
(19.0–23.0) |
80.2 |
|
28–30 |
826 |
15.0 |
(13.6–16.6) |
1,274 |
20.0 |
(18.4–21.7) |
33.3 |
|
Race/Ethnicity |
|||||||
|
White, non-Hispanic |
1,391 |
12.5 |
(11.6–13.6) |
1,791 |
20.0 |
(18.6–21.4) |
60.0 |
|
Black, non-Hispanic |
154 |
9.3 |
(7.5–11.4) |
283 |
18.2 |
(15.2–21.7) |
95.7 |
|
Hispanic |
225 |
13.3 |
(11.0–16.1) |
413 |
20.5 |
(17.8–23.4) |
54.1 |
|
Other/Multiracial |
118 |
13.4 |
(9.7–18.3) |
244 |
19.1 |
(15.5–23.4) |
42.5 |
|
U.S. Census region* |
|||||||
|
Northeast |
305 |
10.2 |
(8.6–12.1) |
429 |
18.2 |
(15.9–20.8) |
78.4 |
|
Midwest |
414 |
12.6 |
(11.0–14.4) |
518 |
20.0 |
(17.6–22.7) |
58.7 |
|
South |
623 |
12.0 |
(10.8–13.2) |
952 |
18.8 |
(17.2–20.7) |
56.7 |
|
West |
557 |
14.6 |
(12.5–17.1) |
859 |
21.9 |
(19.7–24.3) |
50.0 |
|
Health-care coverage |
|||||||
|
Covered |
1,327 |
10.5 |
(9.7–11.4) |
1,838 |
17.1 |
(16.0–18.3) |
62.9 |
|
Not covered |
570 |
19.1 |
(16.9–21.5) |
915 |
27.7 |
(25.2–30.4) |
45.0 |
|
Abbreviations: Pap = Papanicolau; CI = confidence interval. * Northeast: New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont) and Middle Atlantic (New Jersey, New York, Pennsylvania); Midwest: East North Central (Illinois, Indiana, Michigan, Ohio, Wisconsin) and West North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota); South: South Atlantic (Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia), East South Central (Alabama, Kentucky, Mississippi), and West South Central (Arkansas, Louisiana, Oklahoma, Texas); West: Mountain (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming) and Pacific (Alaska, California, Hawaii, Oregon, Washington). † Women receiving a Pap test >36 months before the interview are included in the denominator; these women accounted for 2% of the population in 2000 and 4.2% in 2010. |
|||||||
FIGURE. Prevalence of Pap testing among women aged 18–30 years, by age group — Behavioral Risk Factor Surveillance System, United States, 2000–2010*
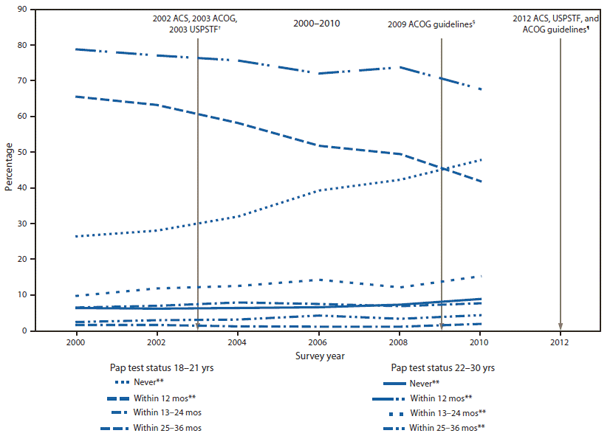
Abbreviations: Pap = Papanicolaou; ACS = American Cancer Society; ACOG = American College of Obstetricians and Gynecologists; USPSTF = U.S. Preventive Services Task Force.
* Data are not shown for women not screened within 36 months of interview (<5%).
† 2002 ACS and 2003 ACOG guidelines recommended Pap testing begin approximately 3 years after onset of vaginal intercourse but no later than age 21 years, with routine screenings every 2–3 years for women aged <30 years with three negative cytology tests. 2003 USPSTF guidelines recommended Pap testing begin within 3 years after onset of vaginal intercourse or at age 21 years (whichever occured first), with routine screenings at least every 3 years.
§ 2009 ACOG guidelines recommended Pap testing begin at age 21 years, with routine screenings every 2 years until age 29 years.
¶ 2012 USPTF and ACS guidelines recommend Pap testing begin at age 21 years, with routine screenings every 3 years.
** Significant change over time (p≤0.05).
Alternate Text: The figure above shows the prevalence of Papanicolaou (Pap) testing among women aged 18-30 years, by age group in the United States during 2000-2010. From 2000 to 2010, increases were observed in the percentages of women aged 22-30 who reported having had a Pap test within the preceding 13-24 (9.8% to 15.3%) and 25-36 months (2.6% to 4.5%).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


