Polio-Free Certification and Lessons Learned — South-East Asia Region, March 2014
, MD1, , MD2, , PhD3, , MBBS4, , MD4, , MPH4, , MD5, , MD5, 6, , MD6, , PhD7, , MD6 (Author affiliations at end of text)
In 1988, the World Health Assembly resolved to interrupt wild poliovirus (WPV) transmission worldwide. By 2006, the annual number of WPV cases had decreased by more than 99%, and only four remaining countries had never interrupted WPV transmission: Afghanistan, India, Nigeria, and Pakistan (1). The last confirmed WPV case in India occurred in January 2011 (2), leading the World Health Organization (WHO) South-East Asia Regional Commission for the Certification of Polio Eradication (SEA-RCC) in March 2014 to declare the 11-country South-East Asia Region (SEAR), which includes India,* to be free from circulating indigenous WPV. SEAR became the fourth region among WHO's six regions to be certified as having interrupted all indigenous WPV circulation; the Region of the Americas was declared polio-free in 1994 (3), the Western Pacific Region in 2000 (4), and the European Region in 2002 (5). Approximately 80% of the world's population now lives in countries of WHO regions that have been certified polio-free. This report summarizes steps taken to certify polio eradication in SEAR and outlines eradication activities and lessons learned in India, the largest member state in the region and the one for which eradication was the most difficult.
Steps Toward Regional Certification
Certification of polio eradication is conducted by WHO regions (1,3–5). The Regional Certification Commission (RCC) is an independent body that certifies a region polio-free when all countries in the region meet three conditions: 1) the absence of indigenous WPV transmission for at least 3 consecutive years, monitored by a sensitive, certification-standard surveillance system; 2) the capacity to detect, report, and rapidly respond to any imported WPV; and 3) documentation of substantial progress toward the eventual laboratory containment (at an appropriate biosafety level) of WPV. Each country has an independent National Certification Committee for Polio Eradication to verify and submit country documentation related to polio eradication activities.
SEA-RCC, comprised of experts in public health, epidemiology, virology, clinical medicine, and related specialties, reviewed the documentation for each SEAR country. The last confirmed indigenous WPV cases in SEAR countries were as follows: Nepal, 2000; Bangladesh, 2000, Burma, 2000; Thailand, 1997; North Korea, 1996; Timor-Leste, 1995 (recognized as independent in 2002); and Indonesia, 1995. Bhutan, Maldives, and Sri Lanka reported their last cases of polio before 1995. India was the final country in the region to successfully interrupt indigenous WPV transmission, reporting the most recent indigenous WPV type 1 (WPV1) case in SEAR in January 2011. Importation of WPV and subsequent spread occurred in four countries after their last indigenous cases: Nepal reported 26 importation-associated cases during 2005–2010, and outbreaks occurred in Indonesia during 2005–2006 (351 cases), Bangladesh in 2006 (18 cases), and Burma during 2006–2007 (11 cases) (Figure 1). In SEAR, the last identified WPV type 2 (WPV2) case occurred in India in October 1999; this was also the last WPV2 case reported globally. The most recent WPV type 3 (WPV3) case in India and the region occurred in October 2010. After careful review of all documentation, SEA-RCC certified that every SEAR country had met the requirements, and the region was declared polio-free on March 27, 2014 (6).
Immunization Activities in India
Using the most recent population-based survey data available, India estimated nationwide coverage with 3 doses of oral poliovirus vaccine (OPV) delivered by routine childhood immunization services to be 70.4% among children aged 12–23 months during 2009–2010. Routine coverage estimates in Bihar (61.6%) and Uttar Pradesh (53.9%), two polio-endemic states, were among the lowest in the country (7). Estimated routine coverage improved substantially from estimated levels reported in 2006 in Bihar (47.6%) and Uttar Pradesh (43.8%).
Supplemental immunization activities (SIAs)† in India were introduced as National Immunization Days (NIDs) in 1995, targeting children aged <3 years. NIDs during subsequent years targeted children aged <5 years. NIDs were reinforced by subnational immunization days and large-scale mop-up activities in endemic and other high-risk areas, as well as by introducing house-to-house vaccination as part of the efforts to identify and vaccinate children who were not being brought to the fixed sites providing OPV during SIAs. Since 2000, during NIDs, more than 2.3 million vaccinators visited approximately 209 million households to vaccinate more than 170 million children aged <5 years. Also, surveillance and monitoring data were used to identify high-risk populations and areas for which innovative strategies were designed and implemented. These included strategies to reach mobile and transitory populations by stationing vaccinators at bus stops and train stations, on trains, and at important road intersections, as well as in markets, in migrant camps, at brick kilns, and at construction sites. Transit teams vaccinated nearly 10 million children in each campaign, more than 100,000 of them while on trains. Innovative strategies also were designed to reach children in access-compromised areas in the Kosi river flood plain in Bihar state and in the traditionally highest-risk areas in western Uttar Pradesh and central Bihar. Monitoring data§ were used to estimate the proportion of children missed in each SIA; since 2010, SIAs in India are estimated to have achieved >95% coverage among targeted children, even in remote areas (2).
India introduced monovalent OPV type 1 (mOPV1) and type 3 (mOPV3) in 2005 (8). Predominant use of mOPV1 greatly reduced the incidence of WPV1 but allowed increased WPV3 incidence until bivalent OPV types 1 and 3 (bOPV) was introduced in 2010 (9). The intensification of the migrant and transit strategies coupled with predominant use of bOPV was associated with a reduction in both WPV1 and WPV3 (Figure 2).
Wild Poliovirus Surveillance in India
Acute flaccid paralysis (AFP) surveillance. To demonstrate that WPV circulation has been interrupted, the global standard in endemic countries for sensitive AFP surveillance is an annual rate of at least two nonpolio AFP (NPAFP)¶ cases per 100,000 children aged <15 years. In India, the national NPAFP rate was 13.9 per 100,000 children aged <15 years in 2012 and 12.5 per 100,000 in 2013. The highest state-level NPAFP rates were in Bihar (34.2) and Uttar Pradesh (21.5) Adequate stool specimen collection in India was 87% nationally in 2012 and 86% in 2013, exceeding the performance standard of 80%.
Environmental surveillance. Systematic (weekly or biweekly) testing of wastewater samples for poliovirus began in Mumbai, Maharashtra state, in June 2001, in Delhi in May 2010, in Patna, Bihar, in April 2011, in Kolkata, West Bengal, in December 2011, and expanded further to include sampling in the states of Punjab and Gujarat in 2013. Both WPV1 and WPV3 were detected in wastewater sampled at Delhi sites during 2010; WPV3 was most recently detected in July 2010, and WPV1 was most recently detected in August 2010. The most recent WPV isolated from wastewater in India was WPV1 sampled in November 2010 in Mumbai. No WPV has ever been isolated from wastewater sampled in Patna, Kolkata, Punjab, or Gujarat. All WPV1 and WPV3 isolates from wastewater during 2010 were closely related to WPV1 and WPV3 circulating in central Bihar state during 2009.
Discussion
The certification of SEAR as polio-free conclusively demonstrated that polio can be eradicated in the most challenging settings where the risks for WPV transmission are highest, namely in countries with 1) high population densities, 2) large birth cohorts, 3) high population mobility, 4) poor sanitation, and 5) tropical/subtropical climates. Although such conditions prevail in parts of several SEAR countries, the magnitude and intensity of risks were greatest in India, especially in Uttar Pradesh and Bihar, which have a combined population of approximately 300 million, a monthly birth cohort of approximately 500,000, frequent migration between and outside the states, and lower per-dose effectiveness of trivalent OPV (8,9). Stopping indigenous WPV transmission in India required sustained commitment at every level of government, the provision of adequate fiscal and human resources, the implementation of innovative strategies and approaches, and private sector engagement. Specific lessons learned about ensuring the success of immunization programs throughout India have been successfully applied to address the challenges to polio eradication activities in other countries and are being used to improve immunization services in India (Box).
Strong surveillance is essential for polio eradication. In India, highly sensitive AFP surveillance was supported by a national network of eight fully accredited laboratories capable of basic and advanced molecular virologic detection methods to provide near real-time genetic information, and supplemented by environmental surveillance at key sites. The surveillance system, including the laboratory component, operated highly effectively, as evidenced by performance that consistently surpassed the WHO-recommended standards for global indicators.
Many national and international partners took part in the effort in India. Multiple funding partners helped to supplement the substantial financial investment made to the polio eradication initiative by the Government of India.** Volunteers and community mobilizers played a huge role in the success of India's eradication efforts, especially to identify the country's newborns and track their immunization status. In particular, local Rotary International members provided volunteers and funding and engaged with local and national officials to advocate for the country's immunization programs, including current efforts to improve the routine immunization of infants and young children. The complementary partnership among government and international polio implementing partners contributed to the success of the India polio eradication program.
1National Polio Surveillance Project, World Health Organization; 2Ministry of Health and Family Welfare, Government of India; 3World Health Organization Country Office; 4Immunization and Vaccine Development Department, South-East Asia Regional Office, New Delhi, India; 5Polio Eradication Department, World Health Organization, Geneva, Switzerland; 6Global Immunization Division, Center for Global Health, CDC; 7Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC (Corresponding author: Steven G. F. Wassilak, swassilak@cdc.gov, 404-639-1867)
References
- Moturi EK, Porter KA, Wassilak SGF, et al. Progress toward polio eradication—worldwide, 2013–2014. MMWR 2014;63:468–72.
- CDC. Progress toward poliomyelitis eradication—India, January 2010–September 2011. MMWR 2011;60:1482–6.
- CDC. Certification of poliomyelitis eradication—the Americas, 1994. MMWR 1994;43:720–2.
- CDC. Certification of poliomyelitis eradication—Western Pacific Region, October 2000. MMWR 2001;50:1–3.
- CDC. Certification of poliomyelitis eradication—European Region, June 2002. MMWR 2002;51:572–4.
- World Health Organization Regional Office for South-East Asia. South-East Asia Regional Certification Commission for Polio Eradication. Report of the seventh meeting of SEARO, New Delhi, India, March 26–27, 2014. Available at http://www.searo.who.int/entity/immunization/documents/en.
- United Nations Children's Fund. Coverage evaluation survey 2009: all India report. Available at http://www.unicef.org/india/1_-_CES_2009_All_India_Report.pdf.
- Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science 2006;314:1150–3.
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010;376:1682–8.
- Global Polio Eradication Initiative. Emergency Action Plan 2012–2013. Annex 1:list of innovations in the India polio eradication program Available at: http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EAP/EAP_annex1.pdf.
* Bangladesh, Bhutan, Burma, Indonesia, India, Maldives, Nepal, North Korea, Sri Lanka, Thailand, and Timor-Leste.
† Mass campaigns conducted during a short period (days to weeks), during which a dose of OPV is administered to all children (generally those aged <5 years) regardless of previous vaccination history. Campaigns can be conducted nationally or in portions of the country (i.e., subnational SIAs).
§ SIA monitoring data are obtained from systematic surveys conducted after every SIA in high-risk areas to identify children aged <5 years who were missed with vaccination.
¶ GPEI sets operational targets for countries with current or recent WPV transmission, both nationally and in each province or state. Targets are NPAFP ≥2 per 100,000 population aged <15 years per year, and adequate stool specimen collection from ≥80% of AFP cases, in which two specimens are collected ≥24 hours apart, both within 14 days of paralysis onset, shipped on ice or frozen ice packs, and arriving in good condition (without leakage or desiccation) at a WHO-accredited laboratory.
** Major international partners include WHO, Rotary International, the World Bank, the Bill and Melinda Gates Foundation, UNICEF, and the governments of Germany, Japan, the United Kingdom, and the United States, including CDC.
What is already known on this topic?
Extensive regions of the world were certified as free from indigenous wild poliovirus (WPV) transmission during 1994–2002. Until 2011, only four countries remained that had never interrupted WPV transmission: Afghanistan, India, Nigeria, and Pakistan. These four countries are located in three World Health Organization (WHO) regions. WPV exportations from these reservoirs have occurred into many countries that had previously interrupted their indigenous WPV transmission.
What is added by this report?
India, a member of the WHO South-East Asia Region, had its last WPV case in January 2011, which was also the last case in the region. In March 2014, the South-East Asia Region was declared to be free from circulating indigenous WPV and became the fourth WHO region to be so certified. About 80% of the world's population now lives in countries of regions that have been certified as polio-free.
What are the implications for public health practice?
Stopping indigenous WPV transmission in India required commitment at every level of government, provision of adequate fiscal and human resources, implementation of innovative strategies and approaches, and engagement with the private sector. Specific lessons learned have been successfully applied to address the challenges to polio eradication activities in other countries and are being used to improve immunization services in India.
FIGURE 1. Number of confirmed polio cases from wild poliovirus (WPV) transmission, by country — World Health Organization South-East Asian Region, 2000–2014*
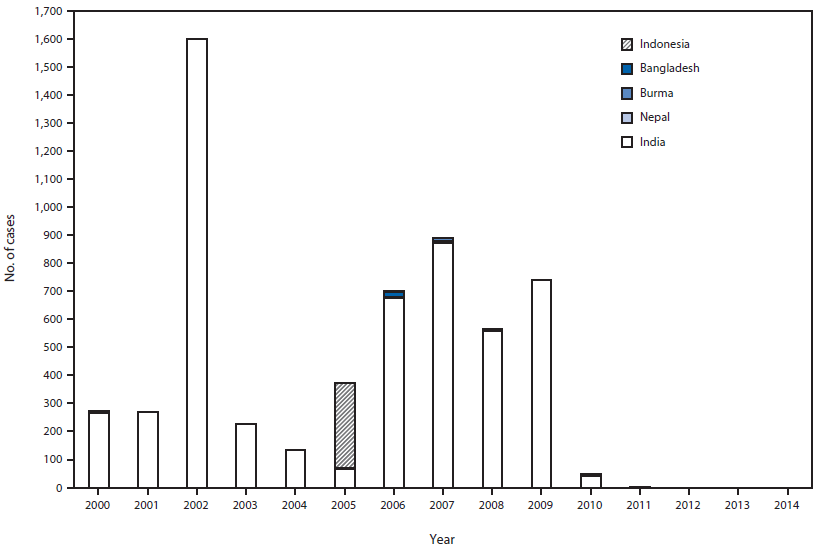
* Cases after 2000 in Bangladesh, Burma, and Nepal were associated with WPV imported from India; a 2005–2006 outbreak in Indonesia was associated with WPV type 1 originating in Nigeria.
Alternate Text: The figure above is a bar chart showing the number of confirmed polio cases from wild poliovirus (WPV) transmission, by country in the World Health Organization South-East Asian Region during 2000–2014. Importation of WPV and subsequent spread occurred in four countries after their last indigenous cases: Nepal reported 26 importation-associated cases during 2005–2010, and outbreaks occurred in Indonesia during 2005–2006 (351 cases), Bangladesh in 2006 (18 cases), and Burma during 2006–2007 (11 cases).
FIGURE 2. Number of polio cases from wild poliovirus types 1 (WPV1) and 3 (WPV3), by month — India, January 2001–January 2011
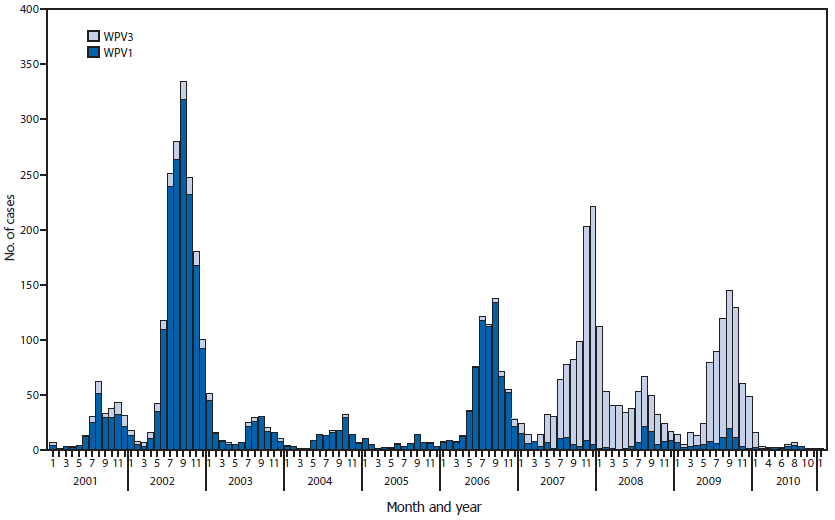
Alternate Text: The figure above is a bar chart showing the number of polio cases from wild poliovirus types 1 (WPV1) and 3 (WPV3), by month, in India during January 2001–January 2011. India introduced monovalent OPV type 1 (mOPV1) and type 3 (mOPV3) in 2005. Predominant use of mOPV1 greatly reduced the incidence of WPV1 but allowed increased WPV3 incidence until bivalent OPV types 1 and 3 (bOPV) was introduced in 2010. The intensification of the migrant and transit strategies coupled with predominant use of bOPV was associated with a reduction in both WPV1 and WPV3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


