Use of Aspirin for Prevention of Recurrent Atherosclerotic Cardiovascular Disease Among Adults — 20 States and the District of Columbia, 2013
, MD1; , MD1; , MD, PhD1; , PhD1 (Author affiliations at end of text)
The effectiveness of regular aspirin therapy in reducing risk (secondary prevention) for myocardial infarction, ischemic stroke, and fatal coronary events among persons with preexisting atherosclerotic cardiovascular disease (ASCVD) is well established (1) and recommended in current guidelines (2). Reported use of aspirin or other antiplatelet agents for secondary ASCVD prevention has varied widely across settings and data collection methods, from 54% of outpatient visits for those with ischemic vascular disease (3) to 98% at the time of discharge for acute coronary syndrome (4). To estimate the prevalence of aspirin use for secondary ASCVD prevention among community-dwelling adults, CDC analyzed 2013 Behavioral Risk Factor Surveillance System (BRFSS) data from 20 states and the District of Columbia. Overall, 70.8% of adult respondents with existing ASCVD reported using aspirin regularly (every day or every other day). Within this group, 93.6% reported using aspirin for heart attack prevention, 79.6% for stroke prevention and 76.2% for both heart attack and stroke prevention. Differences in use were found by age, sex, race/ethnicity, and ASCVD risk status, and state. Most of the state differences were not statistically significant; however, these estimates can be used to promote the use of aspirin as a low-cost (2) and highly effective intervention (1).
BRFSS is an annual telephone survey, conducted by all U.S. states, with guidance and support from CDC. Detailed information regarding the survey can be found online (at http://www.cdc.gov/brfss). In addition to core questions asked by all states, optional BRFSS modules are dedicated to various topic areas. In 2013, 20 states (Arizona, Arkansas, Florida, Georgia, Hawaii, Iowa, Maine, Massachusetts, Minnesota, Mississippi, Missouri, Nebraska, North Carolina, North Dakota, Oklahoma, Oregon, South Carolina, Tennessee, Washington, and Wisconsin) and the District of Columbia opted to include the cardiovascular health module in their surveys. This module included questions about regular (every day or every other day) aspirin use. Respondents who answered "no" when asked about regular aspirin use were subsequently asked whether they had any health problem or condition that made taking aspirin unsafe (e.g., a "stomach condition"). Those who answered "yes," to regular aspirin use were asked additional questions to learn the reason for aspirin use (i.e., to reduce the chance of heart attack, to reduce the chance of stroke, and to relieve pain).*
Participants were classified as having preexisting ASCVD if they reported a history of coronary heart disease or stroke, based on their answers to the following questions: "Has a doctor, nurse, or other health professional ever told you that you had 1) a heart attack, also called a myocardial infarction; 2) angina or coronary heart disease; or 3) a stroke?" Self-reported sociodemographic and descriptive characteristics included age, sex, race/ethnicity, education, and selected ASCVD risk factors (hypertension, diabetes, high cholesterol, current smoking). Prevalence of regular aspirin use was estimated only among those with preexisting ASCVD, stratified by sociodemographic characteristics and number of ASCVD risk factors. Age-standardized prevalence of aspirin use among persons with ASCVD by state was estimated using the 2000 U.S. standard population, based on the following age groups: 18–24, 25–44, 45–64 and ≥65 years (5).
Overall, 175,523 participants aged ≥18 years from 20 states and the District of Columbia responded to the cardiovascular health module, and 21,682 (12.5%) reported a history of coronary heart disease, stroke, or both. From this group, 3,698 (17.1%) were excluded because of missing sociodemographic and ASCVD risk factor data, resulting in a final analytic sample of 17,984. The number of participants ranged from 387 (District of Columbia) to 4,227 (Florida). The median state response rate, calculated according to guidelines of the American Association of Public Opinion Research, was 44%; response rates among states and the District of Columbia ranged from 31% to 59% (6).
Among the eligible respondents with preexisting ASCVD, 70.8% reported regular aspirin use, with 93.6% taking it to prevent a heart attack, 79.6% to prevent a stroke, and 76.2% to prevent both heart attack and stroke (Table 1). Moreover, 14.9% (95% confidence interval [CI]: 13.8%–16.0%) of eligible respondents who reported regular aspirin use and who had ASCVD also reported using aspirin for pain relief, and 4.2% (CI: 3.5%–4.9%) of eligible respondents reported using aspirin for pain relief only. The percentage of aspirin use for prevention of secondary of ASCVD varied by sociodemographic characteristics (Table 1). In general, respondents aged ≥65 years, men, non-Hispanic whites and those with at least two ASCVD risk factors were more likely to use aspirin than other groups.
By state, the age-standardized percentage of regular aspirin use among those with ASCVD ranged from 44.3% (Missouri) to 71.7% (Mississippi) (Table 2) with wide confidence intervals, and most of the observed differences among the states were not statistically significant. No systematic pattern of aspirin use by region was observed (Figure).
Discussion
Overall, 70.8% of adults with preexisting ASCVD in 20 states and the District of Columbia reported regular aspirin use, but differences in aspirin use among various groups were found. Aspirin use for the prevention of recurrent ASCVD is widely promoted across the United States, and is included in national cardiovascular disease prevention programs such as the Million Hearts initiative (7) and Healthy People 2020 (8). Furthermore, CDC-funded state programs to prevent and control heart disease and stroke (e.g., State Public Health Actions to Prevent and Control Diabetes, Heart Disease, Obesity and Associated Risk Factors and Promote School Health† and Well-Integrated Screening and Evaluation for Women Across the Nation [WISEWOMAN]§) support states using strategies for cardiovascular disease and risk factors management outlined in the Million Hearts initiative. These include promotion of the "ABCS" of clinical prevention: aspirin use when appropriate, blood pressure control, cholesterol management, and smoking cessation, as well as promoting healthy environments and encouraging a heart-healthy lifestyle.
Although the overall self-reported prevalence of aspirin use among persons with ASCVD in the BRFSS cardiovascular health module was similar to the 70% recently reported for the 2012 National Health Interview Survey (9), variations in aspirin use were observed in this analysis among geographic areas and sociodemographic groups. Public health practitioners and clinicians can use data from this report to target resources and interventions to those groups with lower use of aspirin. Promotion of adherence to evidence-based practice guidelines and clinical management algorithms upon discharge after cardiovascular disease events, counseling about aspirin use, and implementation of community-based interventions to champion the benefits of regular aspirin use among those eligible are needed to increase aspirin use (10). Further work is needed to assess possible variation in aspirin use at subnational levels and among different risk groups. Although regular aspirin use for pain relief among adults with ASCVD was uncommon, 4.2% of those with ASCVD were using it for pain relief only and not for prevention of heart attack or stroke, receiving the therapeutic benefit of aspirin for secondary ASCVD prevention without realizing it.
The findings in this report are subject to at least five limitations. First, current guidelines recommend aspirin or other antiplatelet medications for prevention of recurrent events; however, data on the use of other antiplatelet medications (as alternatives to aspirin) was not collected by BRFSS. Therefore, the overall use of all antiplatelet medications could not be estimated. Second, the use of aspirin is generally contraindicated after a hemorrhagic stroke (intracerebral hemorrhage and subarachnoid hemorrhage), and BRFSS did not distinguish between hemorrhagic and ischemic stroke. This may partially account for the lower reported prevalence of regular aspirin use for stroke prevention compared with that for heart attack prevention; however, hemorrhagic stroke accounts only for about 10% of all strokes (4). Third, these data are self-reported, and aspirin does not require a prescription for purchase, which might lead to recall bias as well as challenges in identifying the medication. Fourth, because not all states participated in this BRFSS module, the data are not nationally representative. Finally, with relatively small samples of respondents with coronary heart disease or stroke at the state level, confidence intervals for state level estimates were wide.
Although provision of aspirin at discharge following a cardiovascular disease event is high (4), reports using community-based data sources find that aspirin use for secondary prevention is suboptimal. Consistent and timely access to health care services encourages the assessment of ASCVD by clinicians, and community-based interventions to promote aspirin use might reach those persons not likely to interact with health care providers on a regular basis. In addition, interventions targeting specific subgroups, such as those younger than age 65 years, women, and black and Hispanic patients might reduce disparities in aspirin use. The use of this low-cost, effective, and generally safe intervention among persons who have existing atherosclerotic cardiovascular disease is supported by multiple evidence-based guidelines, and current data suggest that there is room for increased use in this population.
Acknowledgments
BRFSS coordinators from states and the District of Columbia; Survey Operation Team, Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
1Division for Heart Disease and Stroke Prevention, National Center for Chronic Disease Prevention and Health Promotion, CDC.
Corresponding author: Jing Fang, jfang@cdc.gov, 770-488-0259.
References
- Baigent C, Blackwell L, Collins R, et al.; Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
- Smith SC Jr, Benjamin EJ, Bonow RO, et al.; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458–73.
- Ritchey MD, Wall HK, Gillespie C, George MG, Jamal A. Million hearts: prevalence of leading cardiovascular disease risk factors—United States, 2005-2012. MMWR Morb Mortal Wkly Rep 2014;63:462–7.
- Mozaffarian D, Benjamin EJ, Go AS, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322.
- Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People statistical notes, no. 20. Hyattsville, Maryland: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2001.
- Behavioral Risk Factor Surveillance System. 2013 summary data quality report. August 15, 2014. Available at http://www.cdc.gov/brfss/annual_data/2013/pdf/2013_DQR.pdf.
- Frieden TR, Berwick DM. The "Million Hearts" initiative—preventing heart attacks and strokes. N Engl J Med 2011;365:e27.
- Healthy People 2020. Heart disease and stroke. Available at http://www.healthypeople.gov/2020/topics-objectives/topic/heart-disease-and-stroke/objectives.
- Fang J, George MG, Gindi RM, et al. Use of low-dose aspirin as secondary prevention of atherosclerotic cardiovascular disease in US adults (from the National Health Interview Survey, 2012). Am J Cardiol 2015;115:895–900.
- Ling G, Ovbiagele B. Oral antiplatelet therapy in the secondary prevention of atherothrombotic events. Am J Cardiovasc Drugs 2009;9:197–209.
* The questions asked were as follows: "Do you take aspirin daily or every other day?" "Do you have a health problem or condition that makes taking aspirin unsafe for you?" "Do you take aspirin to relieve pain?" "Do you take aspirin to reduce the chance of a heart attack?" "Do you take aspirin to reduce the chance of a stroke?"
† Available at http://www.cdc.gov/dhdsp/programs/spha/index.htm.
§ Available at http://www.cdc.gov/wisewoman/.
Summary
What is already known on this topic?
Aspirin and other antiplatelet medications reduce the risk
of cardiovascular events among adults with existing atherosclerotic cardiovascular disease (ASCVD).
What is added by this report?
Among persons with preexisting ASCVD in 20 US states and the District of Columbia, 70.8% reported using aspirin regularly for prevention of heart attack and stroke, and differences were observed in reported aspirin use among certain groups.
What are the implications for public health practice?
Clinical and community-based approaches should be used to increase aspirin use among persons with ASCVD to prevent recurrent heart attacks and strokes, with specific attention to groups reporting lower aspirin use.
FIGURE. Age-standardized percentage of aspirin use among persons with preexisting atherosclerotic cardiovascular disease, by quartile — Behavioral Risk Factor Surveillance System — 20 states and the District of Columbia (DC), 2013
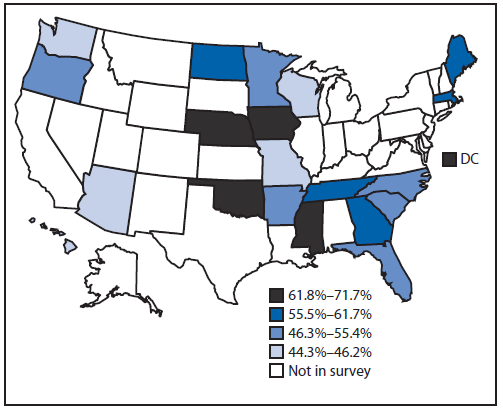
Alternate Text: The figure above is a map showing age-standardized percentage of aspirin use among persons with preexisting atherosclerotic cardiovascular disease during 2013.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


