Measles Outbreak Associated with Vaccine Failure in Adults — Federated States of Micronesia, February–August 2014
, PhD1; , MD2; , MPH5; MPH5; , MD3; , MD2; , MD2; , MPH3; , MD6; , MD7; 8; , MSc5; , MPH2; , MD4; , MD2; , PhD3; , PhD3; , PhD3; , MD3; , MD3; , MD3
On May 15, 2014, CDC was notified of two laboratory-confirmed measles cases in the Federated States of Micronesia (FSM), after 20 years with no reported measles. FSM was assisted by the World Health Organization (WHO), the United Nations Children's Fund (UNICEF), and CDC in investigating suspected cases, identify contacts, conduct analyses to guide outbreak vaccination response, and review vaccine cold chain practices. During February–August, three of FSM's four states reported measles cases: Kosrae (139 cases), Pohnpei (251), and Chuuk (3). Two thirds of cases occurred among adults aged ≥20 years; of these, 49% had received ≥2 doses of measles-containing vaccine (MCV). Apart from infants aged <12 months who were too young for routine vaccination, measles incidence was lower among children than adults. A review of current cold chain practices in Kosrae revealed minor weaknesses; however, an absence of historical cold chain maintenance records precluded an evaluation of earlier problems. Each state implemented vaccination campaigns targeting children as young as age 6 months through adults up to age 57 years. The preponderance of cases in this outbreak associated with vaccine failure in adults highlights the need for both thorough case investigation and epidemiologic analysis to guide outbreak response vaccination. Routine childhood vaccination coverage achieved in recent years limited the transmission of measles among children. Even in areas where transmission has not occurred for years, maintaining high 2-dose MCV coverage through routine and supplemental immunization is needed to prevent outbreaks resulting from increased measles susceptibility in the population.
As an independent country linked to the United States through a Compact of Free Association, FSM receives immunization funding and technical support from the U.S. domestic vaccination program. FSM comprises 607 islands dispersed across 1 million square miles (2.6 million square kilometers) of the western Pacific Ocean. According to the preliminary 2010 census, the population of 102,624 resides in four states: Chuuk (48,651 residents), Pohnpei (35,981), Yap (11,376), and Kosrae (6,616) (1).
As a member country of the WHO Western Pacific Region, FSM is committed to eliminating measles through achieving and maintaining ≥95% vaccination coverage with 2 doses of MCV for each birth cohort of children (2). A single dose of monovalent measles vaccine was introduced in FSM in 1963 and was replaced in 1982 with the measles-mumps-rubella (MMR) vaccine, administered to infants at age 9 months (3). Since the late 1980s, FSM has maintained single-dose MMR vaccination coverage of >70%, most recently exceeding 90% (3). In 1995, a second dose of MMR was introduced. Second dose coverage increased from 50% in 2000 to 85% in 2007, and presently remains around 75% (3). Currently, the first MMR dose is administered at age 12 months, followed by a second dose at age 13 months. Supplementary immunization activities (SIAs), campaigns targeting particular age groups to rapidly increase population immunity, were conducted in Pohnpei in 2011 and in Chuuk in 2004, 2010, and 2013. In Chuuk, SIAs targeted children aged 1–14 years and attained approximately 90% coverage (3). Despite coverage levels <95%, no measles cases had been reported by FSM since an outbreak during 1991–1994, which was associated with 887 cases and 13 deaths (4).
Outbreak
Beginning on February 16, 2014, several patients were evaluated at Kosrae State Hospital for acute onset of fever and rash. No history of travel or specific disease exposures was available in the hospital records. Initial clinical diagnoses were dengue fever or chikungunya. However, during the next several months, as more persons with fever and rash were examined at the hospital, measles was considered as a possible diagnosis. On May 15, FSM reported that serum samples collected from two persons with fever and rash had tested positive for measles-specific immunoglobulin M (IgM) antibodies. During the subsequent investigation, WHO measles case definitions were used (5). A suspected measles case was defined as an illness consisting of fever and a maculopapular rash and cough, coryza, or conjunctivitis in a person of any age, or any illness in a person in whom a clinician suspected measles. Cases were laboratory-confirmed by detection of measles virus nucleic acid from a throat or nasal swab, or measles-specific IgM in a serum sample. Suspected cases were epidemiologically confirmed if they had a rash onset date within 7–21 days of another laboratory-confirmed or epidemiologically confirmed case in the same municipality. Suspected cases with no specimens or with indeterminate laboratory results that could not be epidemiologically linked were considered clinically compatible cases (2). Vaccination history was verified through vaccination records and historical SIA participant lists.
During February 16–June 10, a total of 139 measles cases were detected in Kosrae through febrile rash illness surveillance at the hospital, contact tracing, and a retrospective investigation of earlier fever and rash cases (Figure 1). The first measles cases in Pohnpei were detected on May 12, and during May 12–August 31, 251 cases were reported. The first case in Chuuk was detected on July 24; three cases were reported there during July 24–August 26 (rash onset date could not be confirmed for one patient) (Figure 1). This resulted in a total of 393 measles cases from the three states. No confirmed cases were reported from Yap; 16 suspected cases were investigated and ruled out following negative laboratory results.
Among all 393 cases, 140 (36%) were laboratory confirmed, 244 (62%) were epidemiologically confirmed, and nine (2%) were clinically compatible. Cases were reported for all municipalities in Kosrae and on the main island of Pohnpei, along with one municipality (out of 40) in Chuuk. The median patient age was 24 years (range = 3 weeks–61 years), with 250 (64%) patients aged >19 years. Overall attack rates were highest among infants aged <12 months (56 cases; 22 cases per 1,000 population) followed by adults aged 20–29 years (119; seven per 1,000), and 30–39 years (76; six per 1,000).
Among the 393 measles patients, 306 had vaccination records and the remaining 87 were classified as having unknown vaccination status including 74 adults. Among those with vaccination records, 216 (71%) had received at least one MCV dose before the outbreak, including 169 (96%) of adults aged >19 years. Among adult patients with vaccination records, 123 (70%) had received ≥2 doses of MCV. Among the 90 unvaccinated patients, 54 (60%) were aged <12 months and therefore ineligible for routine vaccination. Among 89 children and adolescents aged 1–19 years with measles, 29 (33%) were unvaccinated; seven (3%) adults were unvaccinated (Figure 2).
Approximately one third of measles patients (n = 124) required hospitalization, and one death was reported (a boy aged 21 months from Pohnpei). Genetic analysis of the nucleotide sequences of measles viruses circulating in Kosrae, Pohnpei, and Chuuk was performed at CDC and identified genotype B3, similar to the B3 lineage reported from a 2013–2014 outbreak in the Philippines and many other countries (6).
Outbreak Response
Vaccination. Each of FSM's four states conducted a mass vaccination campaign to rapidly increase population immunity. In Kosrae, the campaign was conducted during May 22–June 15 and targeted persons aged 6 months–57 years. This campaign included vaccination record checks, and only persons who did not have two documented doses of MCV were vaccinated (4,360 doses administered). The campaigns conducted in Pohnpei and Chuuk targeted all persons aged 6 months–49 years, without checking vaccination records. The campaigns occurred during June 16–September 20 in Pohnpei (29,159 doses administered) and during August 4–December 17 in Chuuk (35,871 doses administered). The campaign in Yap was conducted during June 2–October 12, and 1,998 doses were administered. Persons aged 1–18 years were vaccinated only if they did not have two documented MMR doses, and persons aged 19–49 years were vaccinated without checking vaccination status. On the basis of vaccine doses administered and population census estimates, the percentages of the target populations reached by the campaigns were estimated to be 90% in Kosrae, 95% in Pohnpei, 85% in Chuuk, 42% in Yap, and 87% in FSM overall. The level of immunity achieved through the campaigns appeared to be sufficient to interrupt transmission and stop the outbreak. No cases were reported in FSM after August 31, 2014.
Review of cold chain practices in Kosrae. A limited assessment of vaccine storage equipment and handling practices was conducted at Kosrae Public Health Clinic, the site responsible for vaccine storage and distribution in Kosrae. The clinic provides vaccines to children through statewide campaigns, walk-in visits, and scheduled well-baby appointments, as well as outreach sessions at schools. Some inadequacies in vaccine management were identified, including the use of expired or nonrecommended temperature monitoring equipment, freezing temperatures occurring during vaccine transport, the lack of access to a back-up generator, and the absence of documentation of historical temperature monitoring; the latter precluded the assessment of any past cold chain failures that might have affected the potency of MMR.
Discussion
After 20 years with no reported cases of measles, FSM reported 393 measles cases during February–August, 2014. Delays in detecting the initial measles cases allowed the outbreak to spread and serve as a reminder that measles should be suspected in any case of febrile rash illness, even if the disease has not been seen in the area for years. Approximately two thirds of cases occurred among adults, most of whom had received ≥1 dose of MCV, with many receiving 2 doses. Although measles is typically a childhood disease, outbreaks affecting all age groups have occurred, particularly in populations too small to sustain endemic transmission (4,7,8). However, in this outbreak, the extent of transmission among adults with documented receipt of MMR vaccine, including many with 2 doses, is atypical. Administration of appropriately stored and handled measles vaccine usually produces long-lasting immunity when administered to children aged 12–15 months. Approximately 2%–5% of children who receive 1 dose of MCV fail to develop an appropriate immune response (primary vaccine failure) (9). Most children with primary vaccine failure respond appropriately to a subsequent dose. Secondary vaccine failure refers to waning of vaccine-induced immunity to nonprotective levels. Although distinguishing between primary and secondary vaccine failure is difficult, detection of measles antibody with high avidity in a person with measles suggests secondary failure. Antibody avidity testing is currently being conducted at CDC on specimens collected from cases during this outbreak.
Vaccine failure can result from improper vaccine storage and handling, leading to decreased vaccine potency (10). FSM is a tropical country with multiple vaccine storage challenges, including high ambient temperatures, frequent power outages, and inter-island shipping issues. Although some problems with the vaccine cold chain were identified in Kosrae, it was not possible to assess whether these had affected the potency of vaccines currently in stock because the outbreak response rapidly exhausted existing vaccine supplies. However, few measles cases in children aged <9 years were identified, and most of these occurred in unvaccinated children, which suggests recent cold chain practices were adequate. Historical cold chain lapses are the most plausible explanation for prevalent vaccine failure seen in adults in this outbreak. Earlier problems with vaccine storage and handling might have resulted in suboptimal protection of persons vaccinated decades earlier. Correction of these problems would be expected to lead to protection among persons recently vaccinated. A vaccine effectiveness study was conducted in Pohnpei; when available, the study's findings might determine whether vaccine effectiveness changed over time.
Although most measles outbreaks are caused by a failure to vaccinate all persons who need vaccine, vaccine failure among adults played a major role in this outbreak (4,7,8). Routine vaccination programs and SIAs provided high population immunity among children, with limited transmission in this age group. A series of rapid outbreak response vaccination campaigns increased population immunity and ended the outbreak. The potential role of cold chain lapses in adult vaccine failure highlights the challenges associated with cold chain management in resource-limited settings. These types of challenges might be reduced through availability of more thermostable vaccines. Continued efforts to reach and maintain ≥95% 2-dose MCV vaccination coverage are needed to achieve sustained measles elimination in FSM.
Acknowledgments
FSM Department of Health and Social Affairs; clinical, laboratory, and administrative staff members from the Departments of Health Services of Kosrae, Pohnpei, Chuuk, and Yap states; FSM National and State Immunization Program and staff members.
1Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC; 2Global Immunization Division, Center for Global Health, CDC; 3Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC; 4Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious, CDC; 5Department of Health and Social Affairs, Government of the Federated States of Micronesia; 6Division of Primary Health Care, Pohnpei State, Federated States of Micronesia; 7Department of Health Services, Kosrae State, Federated States of Micronesia; 8Department of Health Services, Chuuk State, Federated States of Micronesia.
Corresponding author: Mark Papania, mpapania@cdc.gov, 404-639-8716.
References
- Federated States of Micronesia Office of Statistics, Budget and Economic Management, Overseas Development Assistance, and Compact Management. FSM 2010 census preliminary results. Federated States of Micronesia; 2015. Available at http://www.sboc.fm/index.
- World Health Organization Regional Office for the Western Pacific. Field guidelines for measles elimination. Geneva, Switzerland: World Health Organization; 2004. Available at http://www.wpro.who.int/publications/docs/FieldGuidelines_for_MeaslesElimination_0F24.pdf.
- Güriş D, Auerbach SB, Vitek C, et al. Measles outbreaks in Micronesia, 1991 to 1994. Pediatr Infect Dis J 1998;17:33–9.
- World Health Organization Regional Office for the Western Pacific. Federated States of Micronesia: country profile-measles elimination. Geneva, Switzerland: World Health Organization; 2015. Available at http://www.wpro.who.int/immunization/documents/measles_country_profile_sep2014_fsm.pdf.
- World Health Organization. Immunizations, vaccines, and biologicals: WHO-recommended surveillance standard of measles. Geneva, Switzerland: World Health Organization; 2015. Available at http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_standards/en.
- World Health Organization. Progress towards measles elimination, Philippines, 1998–2014. Wkly Epidemiol Rec 2015;90:149–59.
- Hyde TB, Dayan GH, Langidrik JR, et al. Measles outbreak in the Republic of the Marshall Islands, 2003. Int J Epidemiol 2006;35:299–306.
- CDC. Measles outbreak and response—Fiji, February–May 2006. MMWR Morb Mortal Wkly Rep 2006;55:963–6.
- CDC. Epidemiology and prevention of vaccine-preventable diseases: measles [Chapter 13]. In: The Pink Book. 13th ed. Atlanta, GA: CDC; 2015. Available at http://www.cdc.gov/vaccines/pubs/pinkbook/meas.html.
- Fowotade A, Okonko IO, Nwabuisi C, Bakare RA, Fadeyi A, Adu FD. Measles vaccine potency and sero-conversion rates among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. J Immunoassay Immunochem 2015;36:195–209.
Summary
What is already known on this topic?
Measles outbreaks occur when the virus is introduced into a population with inadequate immunity, and are almost always caused by failure to vaccinate a high proportion of susceptible persons. Although measles is typically a disease of childhood, persons of all ages can acquire measles, especially in small, isolated, island populations like the Federated States of Micronesia (FSM), where measles has not circulated for many years.
What is added by this report?
After 20 years with no reported measles cases, FSM experienced a moderately large outbreak in 2014 with 393 cases reported. Vaccinated adults accounted for more than half of all cases, demonstrating that in rare circumstances vaccine failure can play a role in measles susceptibility. The cause of the vaccine failure in adults was not determined, although historical cold chain lapses might have reduced vaccine potency and resulted in accumulation of susceptible persons. Moderately high routine 2-dose coverage among children limited transmission in this age group, with the exception of infants who were too young to be vaccinated.
What are the implications for public health practice?
Sustained high 2-dose measles vaccination coverage through routine immunization programs and supplemental immunization activities is needed to prevent the spread of measles outbreaks, even where vaccine failure is a key component of the outbreak. Rapid mass vaccination campaigns can be effective in limiting outbreak spread and should be tailored to the pattern of susceptibility revealed by the outbreak.
FIGURE 1. Number of measles cases by rash onset date* — Kosrae, Phonphei, and Chuuk states, Federated States of Micronesia, 2014†§
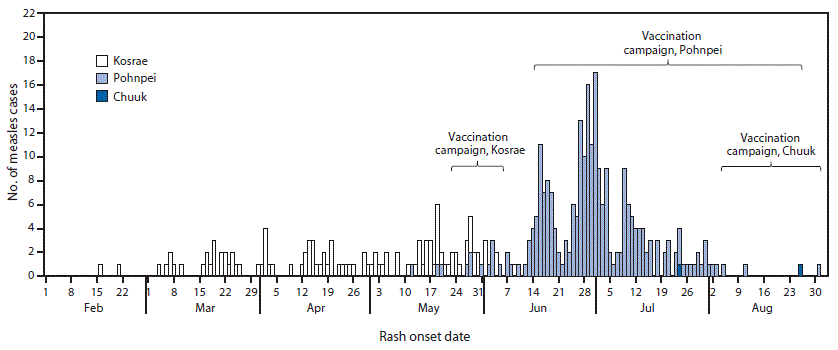
* Rash onset date was missing for 21 cases in Kosrae, two in Pohnpei, and one in Chuuk.
† Vaccination campaign on Pohnpei proper and the lagoon islands occurred during June 16–August 25 and on the outer islands on August 12 and during September 18–20.
§ Chuuk vaccination campaign on the lagoon islands occurred during August 4–October 22 and on the outer islands during September 26–December 17.
Alternate Text: The figure above is a bar chart showing the number of measles cases by rash onset date in the Kosrae, Phonphei, and Chuuk states of the Federated States of Micronesia during 2014.
FIGURE 2. Number of reported measles cases (N = 393), by age group of patients and number of measles-containing vaccine doses — Kosrae, Pohnpei, and Chuuk states, Federated States of Micronesia, February–August 2014
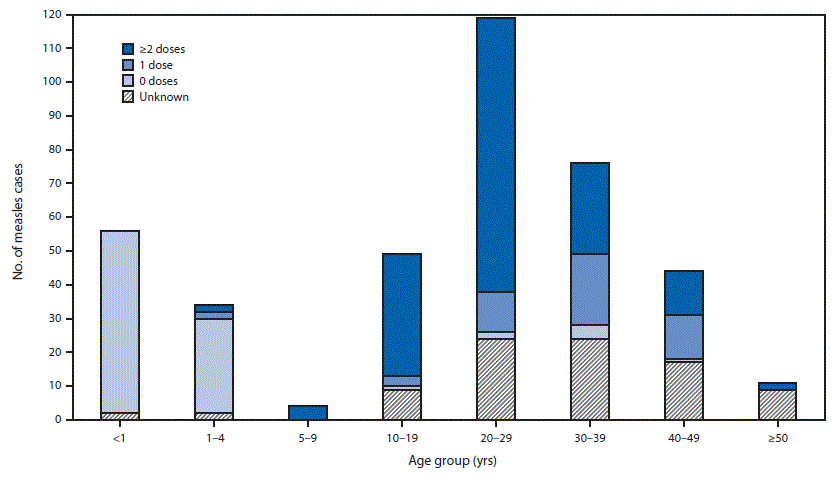
Alternate Text: The figure above is a bar chart showing the number of reported measles cases (N = 393), by age group of patients and number of measles-containing vaccine doses, in the Kosrae, Pohnpei, and Chuuk states of the Federated States of Micronesia during February-August 2014.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


