Vital Signs: Predicted Heart Age and Racial Disparities in Heart Age Among U.S. Adults at the State Level
, PhD1; , MSPH1; , DPT1; , PhD2; , MS1; , MA1; , MD, PhD1; , MD1; , PhD1
Abstract
Introduction: Cardiovascular disease is a leading cause of morbidity and mortality in the United States. Heart age (the predicted age of a person's vascular system based on their cardiovascular risk factor profile) and its comparison with chronological age represent a new way to express risk for developing cardiovascular disease. This study estimates heart age and differences between heart age and chronological age (excess heart age) and examines racial, sociodemographic, and regional disparities in heart age among U.S. adults aged 30–74 years.
Methods: Weighted 2011 and 2013 Behavioral Risk Factor Surveillance System data were applied to the sex-specific non–laboratory-based Framingham risk score models, stratifying the results by age and race/ethnic group, educational and income level, and state. These results were then translated into age-standardized heart age values, mean excess heart age was calculated, and the findings were compared across groups.
Results: Overall, average predicted heart age for adult men and women was 7.8 and 5.4 years older than their chronological age, respectively. Statistically significant (p<0.05) racial/ethnic, sociodemographic, and regional differences in heart age were observed: heart age among non-Hispanic black men (58.7 years) and women (58.9 years) was greater than other racial/ethnic groups, including non-Hispanic white men (55.3 years) and women (52.5 years). Excess heart age was lowest for men and women in Utah (5.8 and 2.8 years, respectively) and highest in Mississippi (10.1 and 9.1 years, respectively).
Conclusions and Implications for Public Health Practice: The predicted heart age among U.S. adults aged 30–74 years was significantly higher than their chronological age. Use of predicted heart age might 1) simplify risk communication and motivate more persons to live heart-healthy lifestyles and better comply with recommended therapeutic interventions, and 2) motivate communities to implement programs and policies that support cardiovascular health.
Introduction
Cardiovascular disease (CVD) is responsible for nearly 800,000 deaths and approximately $320 billion in costs in the United States each year (1). Studies have identified a number of modifiable CVD risk factors, including high blood pressure, smoking, high blood cholesterol, diabetes, and being overweight or obese (1,2). Differences in prevalence of CVD risk factors play important roles in persistent racial, socioeconomic, and regional disparities in CVD morbidity and mortality in the United States (3,4).
To help with the prevention and management of CVD, several multivariable prediction models have been developed to predict the risk for developing CVD based on a person's cardiovascular risk factor profile (2,5,6). Most of these models estimate a person's absolute risk for having a coronary heart disease event or stroke within a certain period (e.g., in the next 10 years). However, predicted absolute risk is an epidemiologic concept that might be difficult for members of the public to interpret and, therefore, its usefulness in motivating lifestyle changes or adherence to recommended therapeutic interventions might be limited (7,8). Moreover, its use might provide false assurance, especially among younger persons whose chronological age might conceal the effects that risk factors (e.g., smoking and uncontrolled hypertension) have on their long-term CVD risk (9).
In 2008, the Framingham Heart Study introduced the concept of heart age (i.e., the predicted age of the vascular system of a person based on his or her cardiovascular risk factor profile) (10). The comparison of heart age to chronological age represents an alternative way to express a person's risk for having a CVD event* and provides information about a person's cardiovascular health that is not clear from the 10-year risk score alone. This method might simplify risk communication and motivate more persons, especially younger persons, to establish heart-healthy lifestyle changes and adhere to recommended treatment strategies (7,8,11). However, no study has provided population-level estimates of heart age and examined disparities in heart age among U.S. adults. This study provides national estimates for heart age, identifies differences between heart age and chronological age, and examines the racial, sociodemographic, and state-level disparities in heart age among U.S. adults aged 30–74 years using 2011 and 2013 Behavioral Risk Factor Surveillance System (BRFSS) data.
Methods
BRFSS is a state-based, random-digit–dialed telephone survey that uses a multistage sampling design to select a state-specific sample from noninstitutionalized U.S. civilian adults aged ≥18 years; a CVD-specific module is conducted in odd-numbered years. Detailed methodology on BRFSS is available at http://www.cdc.gov/brfss. Weighted 2011 and 2013 BRFSS data collected from all 50 states and the District of Columbia were combined to obtain stable estimates; the median combined response rate for each year was 49.7% and 45.9%, respectively. Among 981,660 participants, 403,135 (41%) were excluded, including 234,936 participants aged <30 or ≥75 years, to meet the recommended age range for heart age calculation; 74,834 participants with self-reported coronary heart disease, myocardial infarction, or stroke at baseline; 2,929 pregnant women; and 90,409 participants with missing covariates used for blood pressure prediction, leaving 578,525 participants for analysis.
For estimation of heart age, the sex-specific non–laboratory-based Framingham Risk Score (FRS) was used to estimate the risk for developing CVD in the next 10 years among BRFSS participants, which required the use of the following self-reported attributes: age, current smoking status, antihypertensive medication use, diabetes status, and body mass index (BMI) (10). In addition, because the non–laboratory-based FRS requires the use of systolic blood pressure and BRFSS data do not include measured systolic blood pressure for participants, a previously published method to estimate participants' systolic blood pressure was used (12). In brief, using National Health and Nutrition Examination Survey (NHANES) 2007–2012 data, four sex- and hypertension-status–specific multivariable linear regression models were developed to predict systolic blood pressure. These NHANES-derived parameters were then applied to the comparable variables among BRFSS participants to predict each person's systolic blood pressure. After calculating participants' FRS using their predicted systolic blood pressure, their FRS result was translated to the corresponding predicted heart age, with the upper limit of predicted heart age set at 100 years (10).
Age-standardized and weighted means and prevalence and 95% confidence intervals (CIs) were calculated for participants' chronological age, predicted heart age, the difference between predicted heart age and chronological age (defined as excess heart age). Prevalence of participants whose excess heart age was ≥5 years was calculated by age group (30–39, 40–49, 50–59, and 60–74 years), race/ethnicity (non-Hispanic white [white], non-Hispanic black [black], Hispanic, and others), education (<high school, high school, and >high school), annual household income (<$35,000 or ≥$35,000), and state. Multivariable linear regression models were used to estimate racial disparities in the difference of excess heart age among racial/ethnic groups by age, education, and household income group. Data were analyzed using statistical software that accounted for each surveys' complex sampling design.
Results
Among 236,101 men and 342,424 women, the mean weighted chronological ages were 47.8 and 47.9 years, respectively (Table 1). The corresponding predicted heart ages and excess heart ages were 55.6 and 53.3 and 7.8 and 5.4 years for men and women, respectively (Table 1). Among men, blacks had the highest predicted heart age (58.7 years) followed by Hispanics (55.7 years), whites (55.3 years) and others (54.7 years). Among women, the corresponding values by race/ethnicity were 58.9 years, 53.5 years, 52.5 years, and 52.3 years, respectively. Excess heart age increased with age and decreased as education and household income increased. Overall, approximately 69.1 million (43.7%) U.S. adults aged 30–74 years had excess heart age ≥5 years.† Prevalence of excess heart age ≥5 years was 48.8% among men and 38.5% among women; among both sexes, prevalence was higher among blacks compared with whites, increased with age, and decreased with greater education and household income (Table 1).
Among men, the adjusted difference in excess heart age between blacks and whites was 2.7 years, -1.2 years between Hispanics and whites, and 3.8 years between blacks and Hispanics (Table 2). The corresponding numbers for women were 5.3 years, -1.6 years, and 7.0 years, respectively. The racial differences in predicted excess heart age tended to increase with greater age, education, and household income for blacks compared with whites, but decrease for Hispanics compared with whites (Table 2). For blacks compared with Hispanics, predicted excess heart age tended to increase with greater age, but decrease with greater education and household income.
At the state level, age-standardized excess heart age was lowest in Utah for men (5.8 years) and women (2.8 years) and was highest in Mississippi for men (10.1 years) and women (9.1 years) (Table 3). Similar patterns were observed in the distribution of prevalence of excess heart age ≥5 years by sex and state (Table 3).
Conclusions and Comment
The predicted heart age among surveyed U.S. adults aged 30–74 years was substantially higher than their chronological age. On average, men and women had a predicted heart age 7.8 and 5.4 years older, respectively, than their chronological age, if the selected CVD risk factors were in an ideal range (not smoking, having normal systolic blood pressure (≤120 mmHg) and BMI <25, and not having diabetes). One in two men and two in five women had a predicted heart age ≥5 years older than their chronological age. This finding of high prevalence of excess heart age was consistent with the findings of other studies that have documented only a small proportion of U.S. adults meeting ideal cardiovascular health metrics (13,14).
Among younger adults, predicted excess heart age was higher among men compared with women. For example, among men aged 30–39 years, the average predicted heart age was 3.8 years older than their chronological age, compared with -0.3 years among similarly aged women. This disparity aligns with other findings showing that the mean chronological age of men who have suffered an initial heart attack is about 7 years younger than that of women (65.0 versus 71.8 years) (1). This pattern of greater excess heart age among men was consistent across all the age groups until age 60–74 years, where women's excess heart age surpassed that of men's.
This analysis revealed substantial racial/ethnic disparities in the predicted heart age, with blacks having significantly higher predicted heart age compared with that of other groups. When adjusted for age, education and household income, the excess heart age among black men was 3 or 4 years more than white or Hispanic men, respectively, and among black women was 5 or 7 years more than white and Hispanic women, respectively. The higher predicted heart age among blacks might reflect persistent racial disparities in CVD risk factors,§ especially elevated hypertension prevalence among blacks (3,4).
Predicted heart age differed substantially among states. Among the five states with the highest age-standardized predicted excess heart age for men (Mississippi, Louisiana, West Virginia, Alabama, and Kentucky), the excess heart age was ≥9.7 years, and ≥59.0% of men had excess heart age ≥5 years. Women living in the five states with the highest age-standardized predicted heart age (Mississippi, Louisiana, Alabama, Arkansas, and West Virginia) had an average excess heart age ≥8.0 years, with ≥48.9% of women having excess heart age ≥5 years.
The findings in this report are subject to at least six limitations. First, heart age was calculated using model-estimated systolic blood pressure instead of measured systolic blood pressure. However, use of mean predicted systolic blood pressure in BRFSS participants has been shown to produce a nearly identical 10-year FRS for developing CVD to that of NHANES participants with measured systolic blood pressure (12). Second, the non–laboratory-based FRS that was used to estimate heart age might result in higher predicted heart age from that calculated using laboratory-based FRS estimates (12). Different CVD prediction models, including models developed using data from other cohorts that account for racial/ethnic differences in the effects of risk factors on CVD risk or that incorporate additional CVD risk factors (e.g., physical inactivity), might provide different predicted risk for developing CVD (5,10,15); therefore, the predicted heart age presented in this report should be interpreted with caution. Third, self-reported BMI and diabetes diagnosis were used to estimate heart age among BRFSS participants. Underreporting of BMI is well-documented in BRFSS, and this might underestimate heart age for some participants (16); however, studies indicate that diabetes status by self-report and that based on actual diagnoses have been in substantial agreement in BRFSS and in survey data (17,18). Fourth, BRFSS does not collect self-reported heart failure or peripheral artery disease status, so participants with these conditions were not able to be excluded from these analyses. Fifth, within-state differences in excess heart age likely exist; however, such differences could not be assessed adequately in this study because of limited sample size at the county level. Finally, FRS uses a selected set of CVD risk factors to predict the development of CVD (10). Lifestyle changes, such as reducing consumption of sodium, being physically active, and eating a healthy diet, also play an important role in reducing incidence of CVD but are not included in FRS heart age calculations (19).
Studies suggest that >75% of CVD could be prevented or postponed by controlling and managing specific CVD risk factors through lifestyle changes and/or adherence to recommended treatments (19–21). One important component of the Million Hearts initiative (http://millionhearts.hhs.gov), a national effort to improve access to and quality of care to reduce the incidence of CVD through community and clinical prevention strategies, is to focus on the "ABCS" (aspirin when appropriate, blood pressure control, cholesterol management, and smoking cessation). Greater achievement of the ABCS, in addition to control of other CVD risk factors and reductions in racial and geographic CVD disparities, are critical for meeting the initiative's goal of preventing 1 million heart attacks, strokes, and other CVD-related events in 5 years.
Although traditional absolute CVD risk (e.g., 10-year CVD risk score) should continue to be used by clinicians to inform treatment and management, heart age might be an effective way to communicate individual-level risk for developing CVD and spur action to improve health. One study comparing the effect of using absolute CVD risk versus heart age on participants' risk perceptions and intention to make lifestyle changes suggested that heart age messaging led to significantly higher perceived risk and was more emotionally impactful for participants at higher actual CVD risk levels (7). A randomized intervention trial concluded that communicating CVD risk using heart age versus absolute risk resulted in a greater reduction (-1.5 versus -0.3 year decrease in heart age) in CVD risk over the 1-year intervention period (22). Adopting a healthy lifestyle could have a profound effect on reducing excess heart age. For example, a male smoker aged 50 years with untreated systolic blood pressure of 140 mm Hg, no diabetes, and a BMI of 30, has a predicted heart age of 72 years (74 years for a female with similar characteristics) (Figure).¶ Quitting smoking for 1 year alone would have reduced predicted heart age by 14 years (15 years), reducing systolic blood pressure to 120 mm Hg alone would have reduced predicted heart age by 6 years (10 years), and removing both risk factors would have lowered predicted heart age by 19 years (23 years). At the population-level, the use of predicted heart age might be an effective way to communicate CVD risk, to identify geographic regions and populations most in need of CVD risk factor improvement,** and to stimulate action at the state, county, or community level.
Considerable burden of elevated heart age exists in the United States, and statistically significant racial, sociodemographic, and regional disparities in heart age exist among U.S. adults aged 30–74 years. Use of heart age might simplify risk communication and motivate more persons, especially younger persons, to adopt healthier lifestyles and better comply with recommended therapeutic interventions to prevent heart disease and stroke. Moreover, its use might support public health efforts in geographic areas most at risk for poor CVD outcomes and support the implementation of programs and policies that increase the availability of heart-healthy lifestyle options within communities.
Acknowledgments
BRFSS coordinators from states and the District of Columbia; Survey Operation Team, Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
1Division for Heart Disease and Stroke Prevention, CDC; 2Habit Partners Community Interest Company, London, United Kingdom.
Corresponding author: Quanhe Yang, qay0@cdc.gov, 770-488-8559.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322.
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47.
- Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis 2007;17:143–52.
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation 2005;111:1233–41.
- Siontis GC, Tzoulaki I, Siontis KC, Ioannidis JP. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ 2012;344:e3318.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl 2):S49–73.
- Soureti A, Hurling R, Murray P, van Mechelen W, Cobain M. Evaluation of a cardiovascular disease risk assessment tool for the promotion of healthier lifestyles. Eur J Cardiovasc Prev Rehabil 2010;17:519–23.
- Groenewegen K, den Ruijter H, Pasterkamp G, Polak J, Bots M, Peters SA. Vascular age to determine cardiovascular disease risk: a systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol 2015 [Epub ahead of print].
- Berry JD, Liu K, Folsom AR, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation 2009;119:382–9.
- D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53.
- Anderson TJ, Grégoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67.
- Yang Q, Zhong Y, Ritchey M, et al. Predicted 10-year risk of developing cardiovascular disease at the state level in the U.S. Am J. Prev Med 2015;48:58–69.
- Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012;125:987–95.
- Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012;307:1273–83.
- Allan GM, Nouri F, Korownyk C, Kolber MR, Vandermeer B, McCormack J. Agreement among cardiovascular disease risk calculators. Circulation 2013;127:1948–56.
- Yun S, Zhu BP, Black W, Brownson RC. A comparison of national estimates of obesity prevalence from the behavioral risk factor surveillance system and the National Health and Nutrition Examination Survey. Int J Obes 2006;30:164–70.
- Bowlin SJ, Morrill BD, Nafziger AN, Jenkins PL, Lewis C, Pearson TA. Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol 1993;46:561–71.
- Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol 2004;14:507–16.
- Eckel RH, Jakicic JM, Ard JD, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl 2):S76–99.
- Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: time to end the "only-50%" myth. Arch Intern Med 2001;161:2657–60.
- Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA 1999;282:2012–8.
- Lopez-Gonzalez AA, Aguilo A, Frontera M, et al. Effectiveness of the Heart Age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur J Prev Cardiol 2015;22:389–96.
* A CVD event is the development of coronary heart disease (coronary death, myocardial infarction, coronary insufficiency, and angina), cerebrovascular disease (ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication), or heart failure.
† To determine the number of persons with heart age greater than chronological age, the sex-specific prevalence of adults aged 30–39, 40–49, 50–59, and 60–74 years free from CVD was determined using NHANES 2007–2012 data. Next, these prevalence estimates were applied to the NHANES 2011–2012 age- and sex-specific noninstitutionalized U.S. civilian population counts to determine the number of adults by age category free from CVD during that period. Finally, the BRFSS 2011 and 2013 derived age- and sex-specific heart age prevalence estimates were applied to these population estimates to determine the age- and sex-specific count estimates averaged across 2011 and 2013.
§ Supplementary Tables 1 and 2 (available at http://stacks.cdc.gov/view/cdc/33002) demonstrate the age-standardized distribution of CVD risk factors included in non–laboratory-based FRS heart age calculations, by race/ethnic group and sex.
¶ A heart age calculator is available at http://www.cdc.gov/heartdisease/heartage.htm.
** Supplementary Table 3 (available at http://stacks.cdc.gov/view/cdc/33002) demonstrates the effect of CVD risk factors included in non–laboratory-based FRS heart age calculations on population mean excess heart age estimates stratified by sex and chronological age.
|
Key Points |
|
FIGURE. Excess heart age among U.S. adults without and with diabetes, by sex, chronological age, smoking status, and untreated systolic blood pressure*†
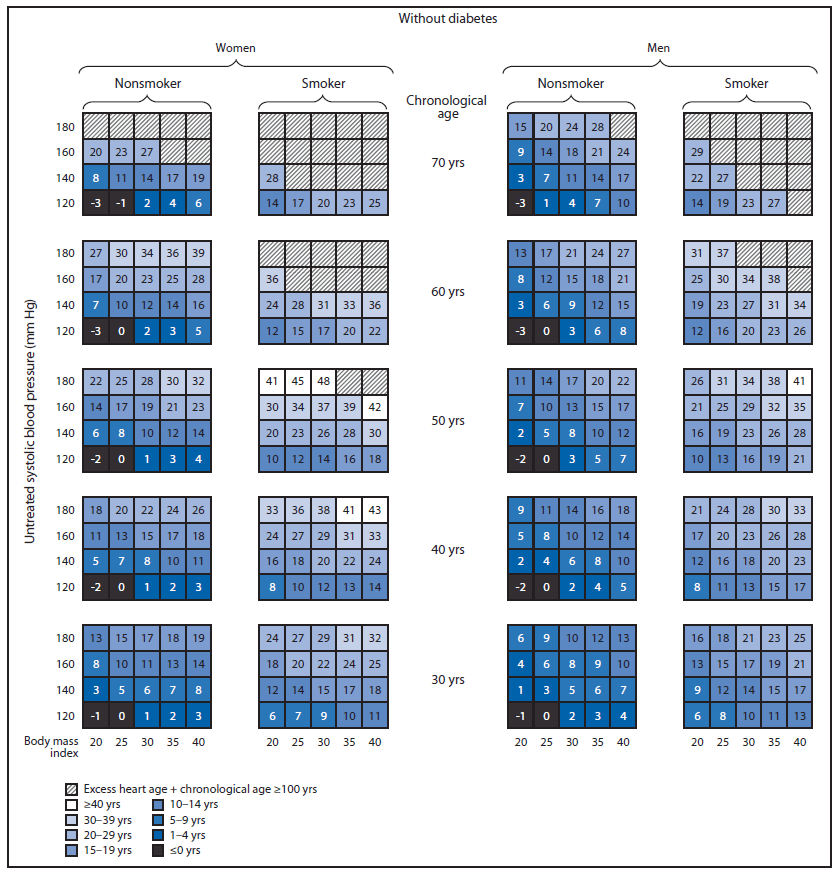
Alternate Text: The figure above is a diagram showing excess heart age among U.S. adults without and with diabetes, by sex, chronological age, smoking status, and untreated systolic blood pressure.
FIGURE. (Continued) Excess heart age among U.S. adults without and with diabetes, by sex, chronological age, smoking status, and untreated systolic blood pressure*†
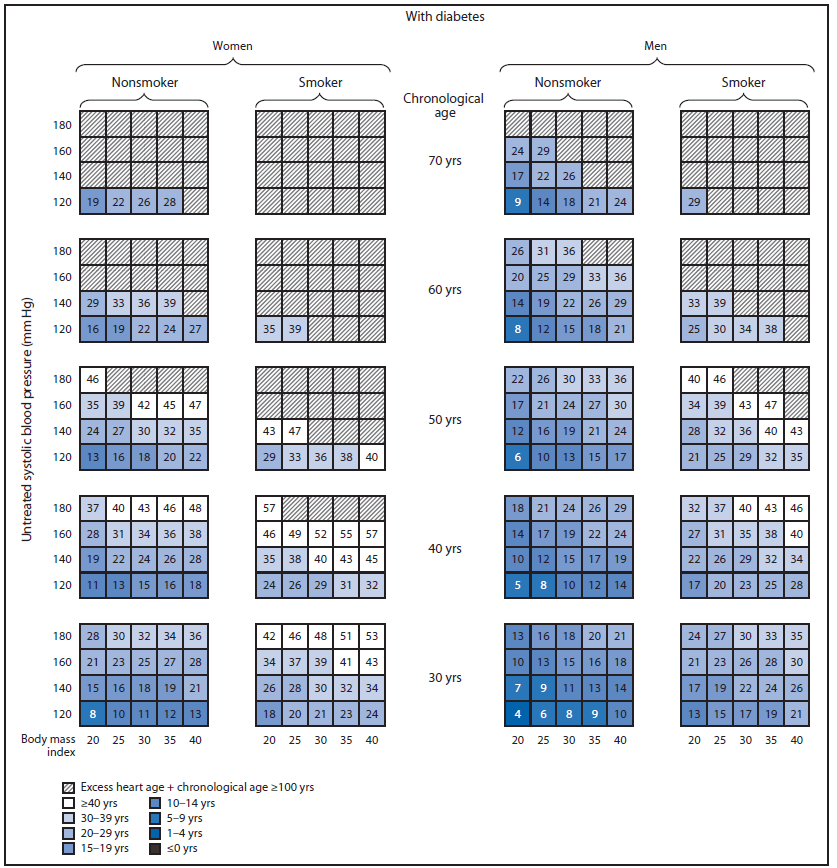
* To determine a person's predicted excess heart age using these charts, follow these steps. Identify the person's 1) diabetes status (without or with diabetes); 2) sex (woman or man); 3) smoking status (nonsmoker or smoker); 4) chronological age (rounded to the nearest value of 30, 40, 50, 60, or 70 years); 5) systolic blood pressure (rounded to the nearest value of 120, 140, 160, or 180 mm Hg); and 6) body mass index (rounded to the nearest value of 20, 25, 30, 35, or 40). The value in the corresponding box is the person's predicted excess heart age. This value can be added to the person's chronological age to determine his or her predicted heart age. For example, a male smoker aged 50 years with untreated systolic blood pressure of 140 mm Hg, no diabetes, and a body mass index of 30, has a predicted excess heart age of 22 years and a heart age of 72 years.
† An upper limit of predicted heart age has been set at 100 years.
Alternate Text: The figure above is a diagram showing excess heart age among U.S. adults without and with diabetes, by sex, chronological age, smoking status, and untreated systolic blood pressure.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


