Vital Signs: Increased Medicaid Prescriptions for Preexposure Prophylaxis Against HIV infection — New York, 2012–2015
, PhD1; , MA, MLS2; , MPS1; , MD, JD3
Abstract
Background: Approximately 3,000 incident cases of human immunodeficiency virus (HIV) infection occur in New York state each year. Daily HIV preexposure prophylaxis (PrEP) with the oral antiretroviral medication Truvada is a key component of New York's plan to end HIV/acquired immunodeficiency syndrome (AIDS) as an epidemic in the state by 2020.
Methods: Prescription data from the New York state Medicaid program from July 2012 through June 2015 were analyzed with an algorithm using medication and diagnoses codes to identify continuous use of Truvada for >30 days, after excluding use for postexposure prophylaxis or treatment of HIV or chronic hepatitis B infection.
Results: During July 2012–June 2013, a total of 259 persons filled prescriptions for PrEP in the Medicaid program. During July 2013–June 2014, a total of 303 persons filled prescriptions for PrEP. During July 2014–June 2015, a total of 1,330 persons filled prescriptions for PrEP, a substantial increase over the previous 12 months. Across all periods studied, 1,708 Medicaid recipients filled at least one prescription for PrEP, most of whom were New York City (NYC) residents, male, aged <50 years, and, for those with available data on race, white.
Conclusions: PrEP use by Medicaid-insured persons increased substantially in the years following statewide efforts to increase knowledge of PrEP among potential prescribers and candidates for PrEP. Other jurisdictions can follow New York state's example by taking similar steps to remove the financial and knowledge barriers experienced by both potential users and prescribers of PrEP.
Implications for Public Health Practice: Although both state and local health department efforts contribute to the availability and use of PrEP, their collaboration enhances the successful implementation of strategies to increase PrEP use. In addition, the decision by the state Medicaid agency to cover PrEP recognizes the long-term benefits of preventing HIV infections.
Introduction
On June 29, 2014, the Governor of New York detailed a plan to end human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) as an epidemic in the state. The goal of this initiative is to reduce the annual number of newly diagnosed cases of HIV infection from an estimated 3,000 to 750 by the end of 2020, thereby achieving the first-ever decrease in HIV prevalence in the state. The three major points of this plan are to 1) identify persons with undiagnosed HIV and link them to care, 2) link and retain persons with diagnosed HIV infection in health care to maximize the likelihood of virus suppression so they remain healthy, and to prevent further transmission of the virus, and 3) facilitate access to preexposure prophylaxis (PrEP) for high-risk HIV-negative persons to prevent their becoming infected and transmitting the virus (1). After the Governor's announcement, a task force including representatives from the community, academia, and state and local governments was formed; the task force produced a set of 37 recommendations and strategies to accomplish the goals of the Ending the AIDS Epidemic initiative (2). This report describes New York state's policy and programmatic efforts to increase the use of PrEP among Medicaid recipients, and to examine changes in the number of recipients filling prescriptions for PrEP during July 2012–June 2015.
In 2009, the New York State Department of Health's (NYSDOH) AIDS Institute staff recognized the potential for PrEP as a biomedical HIV prevention strategy, should the results of clinical trials underway at that time in the United States and internationally demonstrate the efficacy of PrEP to protect against HIV infection, and began to lay the groundwork for implementing PrEP to reduce HIV acquisition among state residents. By 2012, CDC had published information and guidance concerning PrEP (3,4), and the Food and Drug Administration had approved Truvada (a fixed-dose combination of emtricitabine/tenofovir disoproxil fumarate [FTC/TDF]) for daily use by uninfected adults to help protect against acquiring HIV through high-risk sexual contact. The AIDS Institute then drafted and began to implement a strategic plan (Box) to promote comprehensive and integrated PrEP and related services throughout the state within primary care, HIV care settings, and other settings that serve persons at risk for HIV and sexually transmitted diseases. In January 2014, the AIDS Institute posted its Guidelines for the Use of Preexposure Prophylaxis (PrEP) to Prevent HIV Transmission to its clinical guidelines website (5). This was followed in May 2014 by the release of the U.S. Public Health Service's clinical practice guideline for PrEP (6). To ensure that PrEP would be available to high-risk HIV-negative Medicaid recipients, the state's Medicaid program approved coverage of Truvada for PrEP through the program's fee-for-service drug formulary.
As a key component of New York's plan to end HIV/AIDS as an epidemic in the state, expanding access to PrEP among persons at highest risk for HIV infection has the capacity to substantially reduce the number of incident infections. NYSDOH partnered with the New York City Department of Health and Mental Hygiene (NYCDOHMH) in developing elements of the strategic plan. For example, NYCDOHMH developed and distributed a PrEP toolkit for primary care providers, which the state adapted for use in areas outside of NYC. Also, the city and state health departments collaborated on developing an online directory of providers currently offering PrEP, which is publicly available on their respective websites. NYCDOHMH also devoted an issue of its City Health Information series to providing comprehensive health care to men who have sex with men (MSM) (7) and drafted a PrEP provider FAQ sheet (8), both of which are posted on the agency's website, and created a web address to which queries concerning PrEP and postexposure prophylaxis (PEP) can be sent.
Methods
An algorithm was developed and applied to Medicaid fee-for-service claims and encounter data submitted by Medicaid managed care plans that are housed in the NYSDOH Medicaid data warehouse (Figure). This algorithm included diagnosis and prescription drug coding that was intended to monitor the number of HIV-negative Medicaid recipients who filled prescriptions for Truvada for PrEP, as distinguished from those recipients receiving Truvada for PEP or as treatment of chronic hepatitis B infection. For this report, this algorithm was applied to the Medicaid data warehouse to examine the number of persons enrolled in Medicaid anytime during the 3-year period from July 2012 through June 2015 and who filled at least one prescription for PrEP.
Results
The number of Medicaid recipients filling at least one prescription for PrEP increased by 17.0%, from 259 recipients during July 2012–June 2013 to 303 recipients during July 2013–June 2014, and more recently by 338.9%, to 1,330 recipients during July 2014–June 2015 (Table). Across all periods studied, 1,708 Medicaid recipients filled at least one prescription for PrEP, among whom 80.7% reside in NYC and 82.4% are enrolled in Medicaid managed care plans. The proportion of persons filling prescriptions for PrEP who were male increased across the periods studied from 54.8% to 78.0%, although the number who were female more than doubled. Among recipients for whom race was reported, the proportion of Medicaid recipients filling prescriptions for PrEP who were white increased from 34.8% in the earlier period to 65.7% during the most recent period. However, the number of black recipients who filled at least one prescription for PrEP increased by 67.3%.
Recipients aged 18–49 years accounted for 85.8% of Medicaid recipients filling prescriptions for PrEP across all periods reported. Males accounted for 63.6% of these recipients, and among males in this age group for whom race was reported, 50.5% were white and 22.2% were black. Although males accounted for an increasing proportion of recipients aged 18–49 years using PrEP, the proportion who were black decreased by nearly three quarters, from 59.8% in the early period to 14.9% in the more recent period; however, males accounted for 56.6% of blacks in this age group.
Conclusion and Comments
New York state experienced substantial increases in the number of HIV-negative Medicaid recipients initiating PrEP after the state's efforts to raise awareness of PrEP among potential users and increase the pool of PrEP prescribers. The state's efforts to provide information to potential users and prescribers included making PrEP-related materials available through the NYSDOH website, training activities, public forums, and other activities focusing on educating potential prescribers and users.
During the 3-year period ending June 30, 2015, the number of Medicaid recipients initiating PrEP in New York state increased more than fourfold. Those recipients initiating PrEP during this period were mostly male, aged <50 years, and, among those for whom data describing race was available, white. Despite the lack of diagnosis coding indicating whether Medicaid recipients filling prescriptions for Truvada are appropriate candidates for PrEP, there is concern that PrEP might not yet be reaching those who are engaging in high-risk behaviors, especially young black MSM (9).
Although the increase in percentage of Medicaid recipients filling Truvada prescriptions for PrEP during the 3-year period is substantial, the number of persons doing so remains low relative to the number needed to treat in order to achieve the goals of New York state's Ending the AIDS Epidemic initiative. Although no specific target goal for PrEP coverage has been set, recent modeling studies and other studies have projected reductions in the number of HIV infections from implementation of PrEP (10,11). For example, results from the United Kingdom's PROUD study examining the impact on gay men of using PrEP showed an 86% reduction in HIV infections among MSM receiving PrEP, suggesting that for every 13 persons on PrEP, one HIV infection is averted (11). Although it is still early in the state's Ending the AIDS Epidemic initiative, which includes efforts to facilitate access to PrEP, monitoring the outcomes of the various strategies outlined in this report and others will provide opportunities to assess the impact of the efforts to increase PrEP use as well as inform where and to which populations resources should be most appropriately directed.
The findings in this report are subject to at least three limitations. First, because the analysis was based on administrative billing data, the results must be interpreted with caution. The algorithm derived and the database against which it is applied use combinations of diagnosis and prescription drug codes typically submitted by medical providers for billing and payment purposes, and the database does not contain information that would permit calculating an estimate of potential candidates for PrEP. Second, the database and the claims and encounter data that constitute it are subject to data submission errors and omissions (notably data describing the race and ethnicity of the recipients and any indication of risk) and lag times between the provision of services and claims submission and adjudication. Finally, although the intent of this analysis is to determine the number of Medicaid recipients on PrEP, the results present the number of recipients filling a prescription for Truvada but not whether those persons are following the PrEP regimen.
Despite these limitations, the findings suggest benefits derived from New York state's efforts to raise the awareness and knowledge of PrEP among persons at risk for HIV infection and the clinicians who care for them and can appropriately prescribe the regimen for their patients. This report focuses on results achieved among the state's Medicaid population, which accounts for one quarter (25.6%) of the state's population (NYSDOH, unpublished data, 2015). In addition, almost 170 practitioners, health centers, and practices throughout the state have listed themselves in NYSDOH's online PrEP/PEP provider directory (http://www.health.ny.gov/diseases/aids/general/prep/provider_directory.htm), although PrEP and its related services are more widely available.
New York state's response to the availability of PrEP and PEP as a key strategic component of the state's initiative to end HIV/AIDS as an epidemic in the state is illustrative of its efforts to raise awareness about HIV prevention interventions among clinicians and all persons at high risk for becoming infected or transmitting the virus, regardless of health insurance coverage. Recommendations by the Ending the AIDS Epidemic initiative task force and the elements of the AIDS Institute's PrEP strategic plan reflect perceived and reported needs and barriers concerning PrEP. Other jurisdictions can take similar steps to implement interventions appropriate to their populations at high risk for HIV infection or transmitting the virus that remove the financial and knowledge barriers experienced by potential users and prescribers of PrEP.
1Office of Medicaid Policy and Programs, AIDS Institute, New York State Department of Health; 2 Office of the Director, AIDS Institute, New York State Department of Health, 3Office of the Commissioner, New York State Department of Health.
Corresponding author: Franklin N. Laufer, franklin.laufer@health.ny.gov, 518-486-1383
Acknowledgments
Dawn K. Smith and several other reviewers at CDC; Woopill Hwang; Steven Feuerstein, Walter Gibson, State University of New York, University of Buffalo; Janet Zachary-Elkind, Anthony Merola, Barbara Rogler, Office of Health Insurance Programs, New York State Department of Health.
References
- New York State Office of the Governor. Governor Cuomo announces plan to end the AIDS epidemic in New York state. June 29, 2015. Available at http://www.governor.ny.gov/news/governor-cuomo-announces-plan-end-aids-epidemic-new-york-state.
- New York State Department of Health. Ending the AIDS Epidemic in New York State. Available at http://www.health.ny.gov/diseases/aids/ending_the_epidemic.
- CDC. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011;60:65–8.
- CDC. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep 2012;61:586–9.
- New York State Department of Health. Guidelines for the use of pre-exposure prophylaxis (PrEP) to prevent HIV transmission. Available at http://www.hivguidelines.org/wp-content/uploads/2015/10/PrEP-Guidance_10-14-15.pdf.
- CDC. Preexposure prophylaxis for the prevention of HIV infections in the United States—2014: a clinical practice guideline. Atlanta, GA: US Department of Health and Human Services, CDC, US Public Health Service; 2014. Available at http://www.cdc.gov/hiv/pdf/guidelines/PrEPProviderSupplement2014.pdf.
- New York City Department of Health and Mental Hygiene. Providing comprehensive health care to men who have sex with men (MSM). City Health Information 2014;33:29–36. Available at http://www.nyc.gov/html/doh/downloads/pdf/chi/chi-33-4.pdf.
- New York City Department of Health and Mental Hygiene. PrEP provider FAQs. Available at http://www.nyc.gov/html/doh/downloads/pdf/csi/csi-prep-hcp-faq.pdf.
- CDC. HIV among gay and bisexual men. Available at http://www.cdc.gov/hiv/group/msm/index.html.
- Koppenhaver RT, Sorensen SW, Farnham PG, Sansom SL. The cost-effectiveness of pre-exposure prophylaxis in men who have sex with men in the United States: an epidemic model. J Acquir Immune Defic Syndr 2011;58:e51–2.
- McCormack S, Dunn D. Pragmatic open-label randomised trial of preexposure prophylaxis: the PROUD study. Abstract 22LB. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington; February 23–26, 2015. Available at http://www.croiconference.org/sessions/pragmatic-open-label-randomised-trial-preexposure-prophylaxis-proud-study.
|
Key Points |
|
FIGURE. Coding algorithm for extracting Medicaid recipients filling prescriptions for PrEP
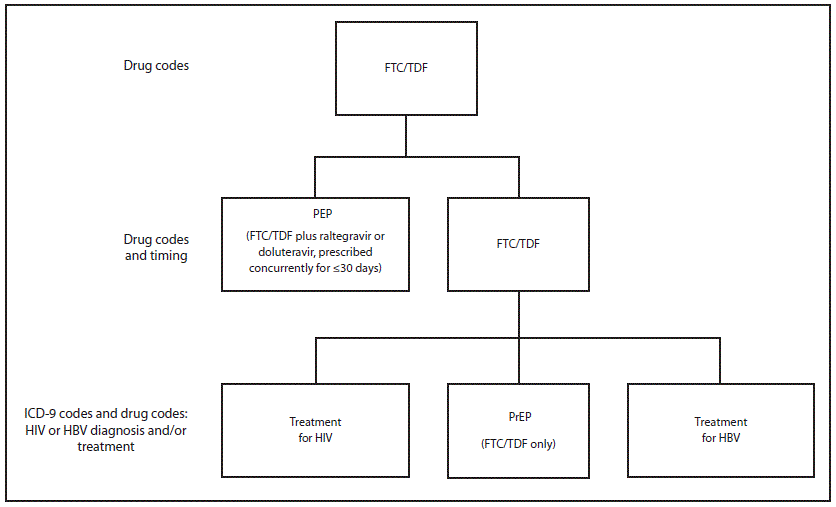
Abbreviations: FTC = emtricitabine; HBV = hepatitis B virus; ICD-9 = International Classification of Diseases, Ninth Revision; PEP = postexposure prophylaxis; PrEP = preexposure prophylaxis; TDF = tenofovir disoproxil fumarate.
Alternate Text: The figure above is a diagram of a coding algorithm used for extracting Medicaid recipients filling prescriptions for PrEP.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


