Competency Guidelines for Public Health Laboratory Professionals: CDC and the Association of Public Health Laboratories
Corresponding preparer: Renée Ned-Sykes, RNed@cdc.gov, 404-498-0125.
Summary
These competency guidelines outline the knowledge, skills, and abilities necessary for public health laboratory (PHL) professionals to deliver the core services of PHLs efficiently and effectively. As part of a 2-year workforce project sponsored in 2012 by CDC and the Association of Public Health Laboratories (APHL), competencies for 15 domain areas were developed by experts representing state and local PHLs, clinical laboratories, academic institutions, laboratory professional organizations, CDC, and APHL. The competencies were developed and reviewed by approximately 170 subject matter experts with diverse backgrounds and experiences in laboratory science and public health. The guidelines comprise general, cross-cutting, and specialized domain areas and are divided into four levels of proficiency: beginner, competent, proficient, and expert. The 15 domain areas are 1) Quality Management System, 2) Ethics, 3) Management and Leadership, 4) Communication, 5) Security, 6) Emergency Management and Response, 7) Workforce Training, 8) General Laboratory Practice, 9) Safety, 10) Surveillance, 11) Informatics, 12) Microbiology, 13) Chemistry, 14) Bioinformatics, and 15) Research.
These competency guidelines are targeted to scientists working in PHLs, defined as governmental public health, environmental, and agricultural laboratories that provide analytic biological and/or chemical testing and testing-related services that protect human populations against infectious diseases, foodborne and waterborne diseases, environmental hazards, treatable hereditary disorders, and natural and human-made public health emergencies. The competencies support certain PHL workforce needs such as identifying job responsibilities, assessing individual performance, and providing a guiding framework for producing education and training programs. Although these competencies were developed specifically for the PHL community, this does not preclude their broader application to other professionals in a variety of different work settings.
Introduction
The national network of governmental public health, environmental, and agricultural laboratories, referred to collectively as public health laboratories (PHLs), is a vital part of the U.S. public health infrastructure. These laboratories perform multiple functions through provision of analytic biological and/or chemical testing and testing-related services that protect human populations from infectious diseases, foodborne and waterborne diseases, environmental hazards, treatable hereditary disorders, and other natural and human-made public health emergencies (1–3). A well-trained laboratory workforce is essential to ensuring that PHLs have the capacity to carry out the critical activities that are needed to safeguard the public's health competently and effectively (4,5).
Studies of the PHL workforce have drawn attention to several concerns about staff training and projected turnover, both of which highlight challenges to maintaining a sufficient number of highly skilled and competent workers. A 2011 national PHL workforce characterization survey found that approximately one third of PHL directors nationally expected 16%–25% of their workforce to retire, resign, or be released in the subsequent 5 years, while 12% anticipated losing 26%–50% of their workforce during that time period (6,7). Approximately 30% of the individual scientific staff respondents expected to continue working in a PHL for <5 years (6). These findings largely reflect workforce demographics, because more than half of scientific laboratory staff were aged >45 years (6). Important recruitment and retention challenges for the PHL workforce also have been identified, including the lack of established progressive job series (commonly termed "career ladders" in the PHL community) for PHL scientists (6–8) and the lack of adequate opportunities for training and professional development (6,7). Indeed, approximately 50% of laboratories reported no, minimal, or only partial capacity to provide continuing education and training to their workers (6,7). Lack of adequate training poses challenges because PHL scientists and managers require a range of scientific, leadership, and managerial development courses, ideally based on core competencies, to function effectively in their positions (9–11).
Multiple national professional organizations, including the Institute of Medicine, the Association of Schools and Programs of Public Health, and the Public Health Foundation/Council on Linkages Between Academia and Public Health Practice (Council on Linkages), among others, have endorsed competency development as a means of strengthening the public health workforce (12–15). Competencies improve the workforce by providing a guiding framework for producing education and training programs, identifying worker roles and job responsibilities, and assessing individual performance and organizational capacity (12–18).
Competencies are action-oriented statements that delineate the essential knowledge, skills, and abilities that are critical to the effective and efficient performance of work (19); competencies should be observable and measurable. Several public health professional disciplines have developed competencies, often through federal partnerships, to address workforce education and training needs (14,15,19–24), and competency-based curriculum development has been suggested as the ideal method for training public health workers and public health students (10,11,13,18,25). In 2009, CDC and APHL collaborated to develop guidelines for biosafety laboratory competency (26), followed by development of this broader set of guidelines for PHL professional competency.
Purpose
The purpose of these guidelines is to outline the knowledge, skills, and abilities that public health laboratory professionals (principally scientists, managers, and leaders) need to deliver the core services of PHLs efficiently and effectively. These guidelines establish core competencies that can help direct workforce development efforts in PHLs in the United States. Because the competencies are universal in nature for many laboratory disciplines, the guidelines also have potential value for laboratories (including those not characterized as public health laboratories) located in both developed and developing nations.
Background
CDC and APHL have engaged collectively in multiple laboratory workforce improvement efforts over the past several years, providing the foundation for the development of these guidelines. More information about these efforts is available at http://www.aphl.org. The 2011 launch of the Laboratory Efficiencies Initiative (27) was intended to assist PHLs in achieving long-term sustainability and resulted in recommendations from multiple forums to focus greater efforts on PHL workforce development. As part of these efforts, APHL collaborated with CDC in 2012 to develop a comprehensive set of competencies that built on APHL's earlier work to draft competencies for PHL professionals across several topic areas (APHL, unpublished draft, 2011). The scope of that project then was expanded to include a broader range and depth of technical and nontechnical competencies, resulting in the guidelines presented in this report. These guidelines for PHL professionals were developed through the engagement of subject matter experts from APHL, CDC, state and local PHLs, federal environmental and agricultural laboratories, clinical laboratories, and academia to ensure appropriate input and vetting.
Methodology
The PHL competencies were developed over a 2-year period through a consensus process involving 108 subject matter experts participating through a variety of committees, workgroups, and teams (Box). In August 2012, CDC and APHL established an 11-member CDC/APHL Steering Committee* to provide direction, guidance, and oversight to the overall competency development process. A 38-member Project Planning Workgroup comprising CDC, APHL, and PHL representatives encompassing a range of expertise (e.g., PHL leadership, informatics, microbiology, and environmental chemistry) was created through consultation with a nationally recognized expert in competency development and was charged with establishing the competency development process. A 2-day meeting of the Project Planning Workgroup (which included the members of the CDC/APHL Steering Committee) facilitated by AlignOrg Solutions was held in Atlanta, Georgia, in October 2012 to define the project scope and target audience, prioritize expectations of stakeholders, discuss methods and criteria for writing competencies, determine the structure of the competencies, and develop a list of draft competency domains to represent the main subject areas in which PHL professionals operate.
Eleven Domain Teams then were established to develop competencies for 14 draft competency domains; an additional domain focused on ethics was developed later in the process. Most team leads and many members were drawn from the Project Planning Workgroup. Each team lead was responsible for making recommendations regarding team membership to the CDC/APHL Steering Committee and APHL staff, who vetted potential members. Domain Team leads were encouraged to recruit a diverse group based on employer type (government and nongovernment, and federal, state, and local), geographic locale of employment, and years and range of experience (laboratory scientists, managers, and leaders). A total of 90 subject matter experts from CDC, state and local PHLs, APHL, academic laboratories, clinical laboratories, the U.S. Department of Agriculture, and others served on the various Domain Teams.
A Development Workgroup created template documents to assist the Domain Teams in constructing their respective competency sets and to ensure uniformity to the process. This Workgroup, which comprised staff from CDC, APHL, PHL, and AlignOrg Solutions, conducted literature reviews and Internet searches to identify related materials, including laboratory association guidelines and reports as well as competency sets for nonlaboratory audiences that had application for PHL functions or specific domain areas (e.g., management, leadership, and workforce training). Although applicable literature was located for eight domain areas (Quality Management System, Management and Leadership, Ethics, Safety, Research, Emergency Management and Response, Workforce Training, and Informatics), limited material was found related to the remaining competency domains. Formal systematic review methods were not used because of the scarcity of available literature, especially regarding laboratory-specific content. In December 2012, the Development Workgroup met to draft a template document for each competency domain, consisting of main competency statements comprising one or more subcompetencies that were further defined by draft responsibility statements at one or more proficiency levels. The Workgroup members used the available literature when applicable but relied principally on their subject matter knowledge and personal work experience as a basis for drafting the template documents.
During January 2013–April 2013, each Domain Team held regular, facilitated conference calls to develop and refine their respective domain competencies, using the template document provided by the Development Workgroup as a starting point. Each team relied on member expertise to arrive at consensus on all competencies, subcompetencies, and proficiency tier statements. The 14 proposed PHL competency domains were mapped against the core functions of PHLs (2,3), the Council on Linkages Core Competencies for Public Health Professionals (14), and the CDC/Council of State and Territorial Epidemiologists Applied Epidemiology Competencies (15) to assess congruence with these materials and to identify gaps in the draft PHL competency domains.
In April 2013, a six-person Synthesis Workgroup was established to review and assess the draft domain documents for gaps and overlaps in content and to harmonize language across domains. During May–November 2013, the Workgroup's efforts focused on developing definitions for each domain, continuing to harmonize content and address gaps and overlaps, developing an online survey tool for the competency validation process, and soliciting reviewers to evaluate one or more competency domains.
Validation Process
The CDC/APHL Steering Committee identified the organizations and agencies it wanted to target for involvement in the competency validation process. Following a solicitation for reviewers, APHL staff contacted 139 potential reviewers on the basis of their knowledge and background in one or more particular domain areas. Of these, 75 were invited and agreed to participate in the review phase. Reviewers were drawn from state and local PHLs, CDC, APHL, clinical laboratory organizations, and food-testing laboratories, as well as former PHL directors and other expert consultants. Fifteen reviewers already had been involved in the competency development process in some capacity and offered to review domains that they did not take part in developing. Reviewers were given 45 days during November 2013–January 2014 to assess the validity of the content for the particular domain(s) reviewed and to provide comments and recommendations for improvement through an online survey tool. The reviewers based their responses on their knowledge and experiences in laboratory practice. In December 2013, an Adjudication Process Workgroup of APHL, CDC, and PHL representatives met to design and establish the process for adjudicating reviewer comments, including a method for each Domain Team to document its response to each comment received. At the end of the vetting period, all reviewer comments were collated and reviewed by APHL staff, and a consolidated listing of comments was forwarded for review to the Domain Teams.
The extensive nature of the comments for the General Laboratory Practice and Emergency Management and Response domains prompted the CDC/APHL Steering Committee to create dedicated teams for each of these domains. Several competencies and subcompetencies for the General Laboratory Practice domain had been extracted previously from the Chemistry and Microbiology domains, with additional content developed by a small work team. The new General Laboratory Practice Domain Team included representatives from the Chemistry, Microbiology, Quality Management System, Research, and Communication Domain Teams. Content for the Emergency Management and Response domain had also been developed initially by the Chemistry and Microbiology Domain Teams. In light of the vetting period comments, the Emergency Management and Response domain competencies were rewritten by a dedicated team that included members from APHL's Public Health Preparedness and Response department and the APHL Public Health Preparedness and Response Committee. Similarly, content for the Ethics domain had been extracted from a number of existing domains, and three Domain Team volunteers formed a new team to examine comments received. All Domain Teams held as many conference calls as needed during January–March 2014 to consider and address the vetting period comments.
In April 2014, a Harmonization of Domains Workgroup met to review major changes that the Domain Teams had made to the draft competencies in response to reviewer comments and to address any remaining overlaps in content and inconsistencies in approach and language among the competency domains. The Workgroup also resolved outstanding issues related to vetting period comments that the Domain Teams were unable to address individually in a consensus process. This workgroup comprised nine Domain Team leads, two CDC/APHL Steering Committee members, the APHL project manager, and an invited representative of CDC. Workgroup members then split into small teams and met via teleconference during April–July 2014 to finalize the competency domains.
In total, the competencies were developed and reviewed by approximately 170 professionals with diverse backgrounds and experiences in laboratory science and public health. The final draft of the competency guidelines was reviewed by the CDC/APHL Steering Committee in October 2014. The final competency guidelines were reviewed and approved by CDC in February 2015.
Guiding Principles
Scope
The competency guidelines were developed specifically for scientists working in PHLs. APHL defines PHLs as governmental public health, environmental, and agricultural laboratories that provide analytic biological and/or chemical testing and testing-related services that protect human populations against infectious diseases, foodborne and waterborne diseases, environmental hazards, treatable hereditary disorders, and natural and human-made public health emergencies (http://www.aphl.org/aboutaphl/aboutphls/pages/default.aspx). Although intended primarily for the continuum of scientist positions from laboratory assistant to laboratory director, these competencies can be used by other PHL staff as well. In fact, all staff (including administrative and support staff, custodial staff, and information technology specialists) can apply competencies within the Security, Safety, Emergency Management and Response, and Ethics domains. Any staff members with responsibilities for data entry, records management, client services, supply services, and other nonbench functions would benefit from application of competencies across additional domains depending on their job function and responsibilities.
Although these competencies were developed expressly for the PHL community, this does not preclude their broader application to a variety of other work settings, because many of the skill sets are similar. Scientists, trainers/educators, and leaders and managers in clinical laboratories, veterinary laboratories, academic and private research laboratories, and other laboratories may use these competencies as a basis for further development of their workforce and local/institutional staff. Laboratories using these competencies should be mindful of federal, state, local, and institutional regulations and standards addressing topics such as safety and security when adopting competencies in practice.
Competencies and Skill Domains
These guidelines were developed on the basis of the Dreyfus Model of Skill Acquisition, which states that five progressive stages of development are associated with skill proficiency (18,28). Learners are able to handle additional responsibility and adjust to different and more complex situations as they gain proficiency. For these competency guidelines, the Dreyfus Model was modified in that four proficiency tiers are used: beginner, competent, proficient, and expert. Descriptions of the proficiency tiers are provided (Appendix A).
Competencies were written by using Bloom's taxonomy as a framework; action verbs describing activities that are observable and measurable were used to signify or relate to a hierarchy of learning and actions (29,30). The hierarchy of responsibilities is based on the science for competency development that has been applied across numerous disciplines. Competencies typically are structured as broad statements that define what is expected of and can be demonstrated by the learner; therefore, specific tasks or methods to achieve the competency at a particular proficiency level are not delineated. Each user is responsible for deciding the needed activities, which might vary substantially because of the diversity that exists within and across public health laboratories. Consequently, competency statements do not refer to or include the frequency with which a responsibility is exercised. Nor, with rare exceptions, do they refer to specific guidelines, standards, or regulations, because these might differ by discipline and type of laboratory. The competencies focus on the knowledge, skills, and abilities required to perform a range of activities in the PHL. The competencies convey the capability for a given behavior or skill, even if the opportunity to perform that skill or behavior is not available (e.g., to serve on national committees or instruct others in policies and processes).
A total of 122 competencies and 519 subcompetencies were identified for the PHL workforce across 15 competency domains: 1) Quality Management System, 2) Ethics, 3) Management and Leadership, 4) Communication, 5) Security, 6) Emergency Management and Response, 7) Workforce Training, 8) General Laboratory Practice, 9) Safety, 10) Surveillance, 11) Informatics, 12) Microbiology, 13) Chemistry, 14) Bioinformatics, and 15) Research (Figure). Full descriptions of these domains and listing of their competencies, subcompetencies, and responsibility statements are presented (Tables 1–15). A number and lettering schema is used to identify domains, competencies, and subcompetencies. Domains are identified by three-letter initials (e.g., QMS), competencies are identified by the domain initials and an overall number (e.g., QMS 1.00), and subcompetencies are further identified through expansion on the competency numbering system (e.g., QMS 1.01 and QMS 1.02).
Three types of domains are included in this competency set: 1) general domains that apply to the responsibilities of all PHL professionals (i.e., Quality Management System, Ethics, Management and Leadership, Communication, Security, Emergency Management and Response, and Workforce Training); 2) cross-cutting technical domains that apply to all laboratory scientists regardless of the scientific discipline in which they work (i.e., General Laboratory Practice, Safety, Surveillance, and Informatics); and 3) specialized domains that are specific to laboratory scientists working in particular scientific disciplines or specialized functional areas (i.e., Chemistry, Microbiology, Bioinformatics, and Research) (Figure). The General Laboratory Practice domain is broadly applicable because it includes general topics pertinent to the laboratory workflow across a wide array of testing areas. Many of these subcompetencies are not duplicated in the specialized domains. Consequently, the General Laboratory Practice domain serves two purposes: to be a companion to each specialized domain and to function as a quasispecialized domain for testing activities not encompassed by the Microbiology or Chemistry domains. Because many topics are relevant to more than one competency domain, the Harmonization of Domains Workgroup decided when to have intentional overlap of particular subcompetencies or topics across domains. For example, the General Laboratory Practice, Research, and Management and Leadership domains include ethics-related competencies although there is a separate Ethics domain.
For all domains, the competencies and proficiency tier statements are context-driven. A given verb is not limited to occurring at only one specific level of proficiency, as the complexity of the described action is determined by the context. In addition, some subcompetencies are similar across domains, in which case users should be mindful of the specific context. For example, sample collection, labeling, and handling are primary functions for both chemistry and microbiology. The subcompetency language for this group of activities is similar in the Chemistry and Microbiology domains, although the specific actions (tasks) needed to achieve each level of proficiency might be different between the testing disciplines.
Each subcompetency outlines a cumulative acquisition of skills, with each successive proficiency tier assuming that a person has acquired the knowledge, skills, or abilities stated in the lower proficiency tier for a given subcompetency. However, the amount of time required for a worker to achieve competency at a particular proficiency tier might be highly variable. In particular, the beginner phase could encompass a very short time frame for some subcompetencies while requiring a longer time for others, depending on the specific action described. Regardless, all beginner tier statements assume that work will be performed under supervision. Furthermore, all statements of the four proficiency tiers are written under the assumption that work will be performed according to standard operating procedures, processes, and policies approved by the administration of the laboratory and in adherence to applicable regulations and accreditation standards and guidelines.
Finally, no recommendation is made that any particular job title or academic degree is required for a particular proficiency tier, nor is the complexity of the knowledge, skills, and abilities for a given proficiency tier the same for all competencies. For example, a PHL scientist could be at the beginner tier for some subcompetencies while being at the competent or proficient tier for others. Similarly, a person might acquire some expert tier competencies early in their career despite not having supervisory or management responsibilities. Many expert competency statements, however, apply to a person occupying a position commensurate with a laboratory director, as significant experience and expertise are required.
Intended Use
These guidelines provide highly structured competencies intended to help ensure a capable, well-trained, and prepared laboratory workforce. The competence of the PHL workforce has a direct impact on the quality of the work output and products required to protect the public's health. Each competency and subcompetency within every domain might not apply to all laboratory staff. The competencies should be tailored and applied to the greatest extent possible to the individual user's situation. Personnel can use the competencies to assess their current skill level and define other areas in need of additional training, with a goal of achieving higher proficiency over time. Laboratory directors and human resources staff might find the guidelines helpful in creating standardized job descriptions, defining progressive job series, recruiting new staff, assessing organizational capacity, and developing performance objectives and appraisals that are aligned with the competencies. Laboratory managers may employ the guidelines as a reference for performance management strategies. The competencies provide a framework for assessing performance and could be used to prepare for certification examinations and for meeting staff qualification requirements specified by governmental laboratory personnel regulations. Finally, educators and training developers may use the guidelines to develop and refine PHL workforce development plans to assess and address training needs through the design of education and training programs.
It is important for users to review the definitions associated with these guidelines (Appendix B). As terms might have different meanings in the context of different laboratory types, the terminology for this project has been standardized to provide more clarity and ease in applying the competencies to practice settings. In situations in which glossary terms contain more than one possible definition, the particular definition applicable to a domain is footnoted for that domain.
Dissemination
CDC and APHL plan to disseminate these guidelines broadly to a variety of stakeholders, including, but not limited to public health laboratories, clinical laboratories, academia, and laboratory scientist professional organizations such as the American Society for Clinical Pathology, the American Society for Clinical Laboratory Science, the Association of Food and Drug Officials, the Association of American Feed Control Officials, the Clinical Laboratory Management Association, and the Clinical and Laboratory Standards Institute, some of which participated in the competency validation process. The competencies will be presented at meetings of public health practitioners and laboratory professionals. The guidelines also will be displayed on APHL's website (http://www.aphl.org).
The next stage of this PHL competency project will focus on the development of tools and resources to aid in guideline implementation. These might include sample competency-based job descriptions and examples of ways professionals can demonstrate competency in a specific area; highlight case study examples of competencies in use; and provide models for developing training and fellowship programs tied to performance metrics and competencies. For example, the Emerging Infectious Diseases Fellowship Program, which is sponsored by APHL and CDC, could use guidance in the toolkit to integrate these competencies into a training program similar to the manner in which the CDC-sponsored Epidemic Intelligence Service program (31) integrated the Applied Epidemiology Competencies (15). Tools and resources to support implementation will also be displayed on APHL's website (http://www.aphl.org) as they are developed.
CDC and APHL also aim to develop a sustainability plan to evaluate the adoption, use, and need for revision of these guidelines periodically. The Council on Linkages (14) and the Clinical and Laboratory Standards Institute (32) provide models for competency and guideline revision that could support this process.
Quality Management System Competency Guidelines
Purpose statement: The competencies in Quality Management System (QMS) address the knowledge, skills, and abilities required for developing a laboratory's culture of quality (Table 1). The essential elements integrate operations, services, and infrastructure into a system that meets applicable regulatory standards, professional guidelines, and customer requirements for ensuring and maintaining quality and continually improving laboratory services.
Introduction: QMS is a systematic approach for ensuring the consistent quality of the tests performed, the products created, the data generated, and the results reported. Operating within a quality system meets the needs and requirements of public health laboratories as well as the expectations of partners, stakeholders, and users (internal and external customers). A QMS is more than quality assurance and quality control — it also includes all the business processes of a laboratory that are required to ensure quality. Adhering to quality standards for laboratory operations helps laboratories generate consistent, reliable, and reproducible data and results.
As the first responsibility of the public health laboratory staff is to provide quality testing and services to support the health of the public and meet the many needs of their customers, this demand for quality is superimposed on all aspects of laboratory operations. As such, a quality management system is the foundation for every other activity within this competency set.
Notes: The structure for this domain is based upon the 12 quality system essentials (33). Multiple additional sources were identified as support documents for this domain (34–40). As the foundational domain for these guidelines, all other competencies should be viewed within its context. However, this domain is systems-oriented. Other domains contain quality-related subcompetencies that address "bench-level" quality indicators and activities and not the creation, maintenance, and evaluation of a quality management system as presented here. The verb "oversees" is used extensively in the Expert level. In this context, "oversees" is a broad term that comprises the many functions related to the management of policies, processes, and procedures to include creation, design, development, directing, monitoring, evaluation, and collaboration.
Ethics Competency Guidelines
Purpose statement: The competencies in Ethics address the knowledge, skills, and abilities needed to fulfill basic responsibilities to perform in a collegial and ethical manner within a laboratory setting (Table 2). Ethical professional and scientific behaviors are essential when working in the public health laboratory to help ensure scientific integrity and sustain effective relationships with stakeholders and the public.
Introduction: Ethics are principles or a set of values held by a person or group, i.e., the rules or standards governing the conduct of a person or the conduct of the members of a profession. These principles and rules include characteristics such as personal accountability, maintaining confidentiality, and ensuring the accuracy of testing results. These vital but sometimes unspoken values, standards, and resulting professional and scientific codes of conduct are critical to establishing and maintaining a collegial environment in which scientific integrity is held in the highest regard.
To carry out its mission, the public health laboratory must earn and maintain the public's trust. As diligent stewards of that trust and of public funds, all public health laboratory staff should act decisively and ethically in service to the public's health. Laboratory staff should apply ethical principles in all aspects of their work, including respecting their colleagues, customers, and populations they serve. Individual laboratory staff members should apply ethical principles in decision-making to all aspects of their job performance and take responsibility for outcomes associated with their decisions. Ethics must exist at every level in the organization; and it must be championed by every staff person, not just leadership.
Notes: Multiple sources were identified as support documents for this domain (41–45). This domain is intentionally broad and includes examples of general and scientific ethics and practices in the glossary. It is the responsibility of each organization to further identify and detail the professional and scientific values and characteristics important to them. The General Laboratory Practice, Research, and Management and Leadership domains also include ethics-related competencies.
Management and Leadership Competency Guidelines
Purpose statement: The competencies in Management and Leadership address the knowledge, skills, and abilities related to managing staff (supervision), the science and practice of achieving results using available resources (management), and the process of influencing the actions of a person or group to attain desired objectives (leadership) (Table 3).
Introduction: Management and leadership are distinct and complementary roles, both of which are necessary for the success of an organization. They can be distinguished in a number of ways (46). Leadership establishes the purpose and strategic direction of the organization. Leading involves innovating, influencing, and motivating. Leadership asks "what" and "why" and mainly works with persons and their interrelationships. Management establishes the systems and processes of the organization. Managing involves administering, planning, organizing, and coordinating. Management asks "how" and "when" and mainly works with systems, processes, mechanisms, models, and structures. Leadership challenges and improves accepted policies and processes and ensures alignment with the mission and vision of the laboratory (i.e., strives to do the right things). Management works within accepted administrative policies and processes to accomplish the mission and vision of the laboratory (i.e., strives to do things right). Effective management and leadership are both critical to accomplishing the core functions of public health laboratories (2,3).
All staff members require a certain degree of management and leadership skills. The scope of work performed by public health laboratories is complex and, therefore, requires staff members who have the crucial leadership and management knowledge and skills to be effective in such an environment. Public health laboratories have a great need to develop these skill sets, as there is a severe and continuing shortage of scientists qualified to assume management and leadership positions. This situation is made more challenging because staff members are rarely provided formal training in these areas through degree, fellowship, or other programs.
Notes: Multiple sources were identified as support documents for this domain (4,10,11,14,15,35,37,46,47), which supports all other domains in these guidelines. This domain is intended for all staff, not just those with managerial or leadership positions or job titles. It is sometimes difficult to separate a skill, behavior, or process as belonging exclusively to either management or leadership. As such, leadership subcompetencies are interwoven throughout the domain and are not limited to those within the Leadership competency (MLD 5.00). Ethics-related competencies are included in this domain that correlate with competencies found in the Ethics domain.
Communication Competency Guidelines
Purpose statement: The competencies in Communication address the knowledge, skills, and abilities necessary to disseminate information in a clear and concise manner appropriate to a given audience (Table 4). Communication might occur in writing, orally, or nonverbally, and it might take place in person or through electronic means.
Introduction: Communication is the application of written, verbal, and nonverbal methods and resources, either in person or through available technologies, to convey information. Although transmission of information is critical, assurance the information is accurate, clear, tailored to the audience, and prepared with linguistic aptitude and cultural sensitivity is equally important.
Effective internal and external communication is necessary for the optimal operation of the public health laboratory. Internal communication between staff is essential to satisfy the organization's goals and quality management system. External communication is necessary to disseminate public health information and to highlight the importance of laboratory contributions in support of public health. Public health laboratories are often called upon to convey the mission, operational features, and test services (the "why," "how," and "what") of their laboratory. These tasks involve engaging traditional and nontraditional partners and are critical to ensuring continued interest and support of the public health laboratory system.
Notes: Multiple sources were identified as support documents for this domain (14,15,48,49). Communication skills are pervasive throughout other domains, including skills such as writing and instructing. Communicating or reporting test orders and results are not covered here but in the General Laboratory Practice, Chemistry, and Microbiology domains.
Security Competency Guidelines
Purpose statement: The competencies in Security address the knowledge, skills, and abilities necessary to ensure a secure, protected working environment that meets or exceeds applicable regulatory requirements and guidelines (Table 5).
Introduction: Security is a compilation of elements that include physical, operational, information, and staff protection with the intent to safeguard personnel and to protect assets and data from unauthorized access, misuse, loss and/or theft. This is accomplished through the implementation of a comprehensive security management system, founded on accepted practices, that ensures that operations are carried out in an environment that is secure and protected at all times. Each person must consistently carry out their responsibilities to ensure the effective application of security practices.
To meet its mission, the public health laboratory must ensure the security of the environment, infrastructure, staff, and of the samples and sensitive information with which it is entrusted. Thus, the knowledge, skills, and abilities included in this domain must be integrated into all technical and nontechnical aspects of staff members' job performances.
Note: Multiple sources were identified as support documents for this domain (50–52).
Emergency Management and Response Competency Guidelines
Purpose statement: The competencies in Emergency Management and Response address the knowledge, skills, and abilities needed to mitigate, prepare for, respond to, and recover from laboratory-specific emergency events and situations (Table 6).
Introduction: Emergency Management and Response is a four-phase process involving mitigation, preparedness, response, and recovery for emergency events and situations that have a direct impact on laboratory operations and surge testing. This domain recognizes the public health laboratory's mandate to provide emergency response support to external partners. Emergency management and response encompasses events such as natural disasters or public health emergencies, facility or operation failures, in addition to the public health responsibility to detect and respond to real or potential biological, chemical, or radiological threats.
Public health laboratory staff members are responsible for the recognition, response, and management of emergency events and situations directly impacting laboratory operations and surge testing. Staff members provide outreach, training, and communication with the sentinel clinical laboratories, first responders, and other stakeholders as a critical role in jurisdiction-wide emergency management and response.
Notes: Multiple sources were identified as support documents for this domain (21,26,51,53–57). This domain is intended to be used in conjunction with the Safety and Communication domains and is based on the Federal Emergency Management Agency's (FEMA) mission areas for national preparedness (55). Critical activities in each phase frequently overlap. This domain does not address accidents, spills, or other similar occurrences within the laboratory, which are included in the Safety domain.
Workforce Training Competency Guidelines
Purpose statement: The competencies in Workforce Training address the knowledge, skills, and abilities needed to train public health laboratory professionals (Table 7). This includes the design, development, implementation, and evaluation of all types of training.
Introduction: Workforce Training is a process that uses principles of adult learning and instructional design to develop, manage, deliver, and evaluate internal and outreach education and training activities. Although training services and resources can be accessed externally, management should also support internal activities to ensure staff members possess the skills and knowledge to carry out their responsibilities in all aspects of their job performance. This domain provides guidance to staff members on subject matter expertise and project management for the development and delivery of training.
As persons are an organization's most valuable asset, having well-trained staff members at all functional levels improves organizational performance and ensures the success of the laboratory in providing services to address public health concerns.
Notes: Sources were identified as support documents for this domain (36,58). This domain is intended for the general laboratory scientist and not solely for education or training specialists or subject matter experts. The competencies apply to all types of training modalities.
General Laboratory Practice Competency Guidelines
Purpose statement: The competencies in General Laboratory Practice address the knowledge, skills, and abilities needed to fulfill basic responsibilities for performing sample analyses within a public health laboratory setting (Table 8).
Introduction: General laboratory practice is the set of foundational knowledge and capabilities needed for the testing of samples across the wide spectrum of scientific and technical activities of public health laboratories. As these practices can be applied in many areas of analysis, they have been consolidated into this domain to minimize, but not eliminate, repetition across the specialized domains and to create a domain that covers testing not specifically encompassed by the Chemistry or Microbiology domains.
These broad practices are central to the performance of laboratory testing. Laboratory scientists, regardless of their specific area of scientific or technical expertise, rely on these skills to accomplish the array of testing in public health laboratories.
Notes: Sources were identified as support documents for this domain (59,60), which is intended for both general and specialized laboratory scientists. This domain is meant to be used in conjunction with specialized domains such as Microbiology, Chemistry, and Research since it includes technical practices not addressed in those domains. The verb "oversees" is used extensively in the Expert level. In this context, "oversees" is a broad term that comprises the many functions related to the management of policies, processes and procedures to include creation, design, development, directing, monitoring, evaluation, and collaboration.
Safety Competency Guidelines
Purpose statement: The competencies in Safety address the knowledge, skills, and abilities necessary to ensure a safe working environment that meets or exceeds applicable regulatory requirements and guidelines (Table 9).
This domain comprises five subdomains:
- Potential Hazards, which addresses the knowledge, skills, and abilities needed to recognize potential hazards within a given laboratory setting;
- Hazard Control, which addresses the knowledge, skills, and abilities needed to support and maintain a health and safety management system to control or prevent workplace hazards;
- Administrative Controls, which addresses the knowledge, skills, and abilities needed to develop a laboratory safety program that is compliant with regulatory, accreditation, and licensing requirements;
- Communication and Training, which addresses the knowledge, skills, and abilities needed to ensure staff members are informed of all safety hazards through effective communication and the provision of related education and training; and
- Documents and Records, which addresses the knowledge, skills, and abilities needed to document activities related to safety policies, processes, and procedures.
Introduction: Safety focuses on the occupational and personal safety of staff members and the environments in which they work. A culture of safety encourages reporting of actual and potential situations which might place staff members and others at risk, openly assesses those risks, and implements redundant systems to keep risk to the absolute minimum. It is essential that leadership and management staff members ensure a comprehensive safety culture for those working in the public health laboratory.
A safety culture is fundamental to ensuring the protection of the laboratory facility, its staff, and the surrounding environment from hazards and risks related to laboratory operations and services. Safety is the background against which all staff members must perform all aspects of their job. A culture of safety recognizes that to err is human, and establishes procedures and processes to minimize errors and avoid harm. To be effective, all staff members are expected to be part of the culture of safety.
This domain is based on the 2011 "Guidelines for Biosafety Laboratory Competency" (26), with the content revised and restructured to fit within this comprehensive set of public health laboratory competencies. This domain supplements and expands upon the 2011 Guidelines. Some reformulation of concepts from the former "Midlevel" and "Senior level" tiers were introduced here to ensure a wider breadth of bench-level and managerial responsibilities. However, the 2011 Biosafety Laboratory Competencies include critical task-level details that could not be captured here due to the directives for competency development that were adopted. The 2011 Guidelines are, therefore, an important companion to this domain.
Note: Multiple sources were identified as support documents for this domain (26,51,52,61–74).
Surveillance Competency Guidelines
Purpose statement: The competencies in Surveillance address the knowledge, skills, and abilities required for the collection and analysis of data to support public health decision making to ensure the health of the community (Table 10). This includes continuous laboratory testing, data compilation, and data dissemination on infectious organisms, chemical analytes, radiological materials, and evidence of hereditary anomalies.
Introduction: Surveillance is the continuous, systematic collection, analysis, and interpretation of health-related data needed for the planning, implementation, and evaluation of public health practice (75). With surveillance, the spread of disease (i.e., any condition that causes injuries, disabilities, disorders, syndromes, infections, or symptoms) is monitored to establish patterns of progression to predict, observe, and minimize the harm caused by the disease. Well-developed surveillance capacity is the foundation on which health departments detect, evaluate, and design effective responses to public health threats. Laboratory information and services are essential to public health surveillance, as the collection, validation, analysis, interpretation, dissemination, and use of laboratory-generated results are crucial to target public health prevention and ensure the health of communities. Public health laboratory scientists and epidemiologists need to work closely to ensure effective population-based disease control and prevention. Effective laboratory reporting to epidemiologists, providers, or other submitters also requires an electronic laboratory reporting (ELR) system that is interoperable with electronic health records and notifiable condition reporting for both care and surveillance.
The public health laboratory plays a unique role in public health surveillance by providing crucial information on the appropriate samples and testing methods, by identifying harmful substances and agents, and by providing the ability to investigate and communicate unusual findings. It is essential for laboratory staff members to understand both their individual role and the laboratory's role in surveillance, testing, reporting, and disease and exposure monitoring.
Notes: Multiple sources were identified as support documents for this domain (15,75–77). Because all public health laboratory testing has a direct or indirect impact on surveillance, this domain is intended for all staff members and not just for persons involved in dedicated surveillance activities.
Informatics Competency Guidelines
Purpose statement: The competencies in Informatics address the knowledge, skills, and abilities needed to systematically apply information science, computer science, and information technology to support public health practice, research, and learning (Table 11).
Introduction: Informatics is a broad field encompassing information science, information technology, algorithms, and social science. In addition to electronic recordkeeping and automated data management, informatics includes such activities as test analyses, clinical decision support, messaging, and knowledge management. Once thought of as a support function, the delivery of laboratory informatics services has now evolved to be a mission-critical and central component of laboratory operations.
Informatics is critically important to the public health laboratory's role in protecting the public from infectious diseases, environmental dangers, and other health threats. Public health laboratory informatics must be cross-cutting and interoperable to support a nationally integrated electronic laboratory reporting (ELR) system and electronic health record (EHR) system. Since all laboratories must rely on informatics capabilities and often have limited access to informaticians or informatics specialists, it is essential that all staff members maintain varying levels of informatics competencies.
Notes: Multiple sources were identified as support documents for this domain (20,78–81). In particular, the competencies defined in this domain are based on the content and framework of a 2013 comprehensive public health laboratory informatics self-assessment tool (78). In turn, this tool was framed on an earlier document (79) outlining consensus on the business requirements of laboratory information management systems. Some of the competencies provided in these guidelines, particularly the Expert level, might appear beyond the reach of the typical bench scientist. While laboratory scientists might initially have competencies limited to the Beginner or Competent level, a long-term goal is to ensure that public health laboratories have within their ranks scientists with competencies at the Proficient and Expert levels. This domain includes paper systems as part of the laboratory information system.
Microbiology Competency Guidelines
Purpose statement: The competencies in Microbiology address the knowledge, skills, and abilities needed to safely and securely detect, identify, and report infectious agents of concern to the public while following the laboratory path of workflow (Table 12).
Introduction: Microbiology is the scientific study of microorganisms and infectious agents as applied to the diagnosis, treatment, and prevention of disease, disability, and death. Microbiology includes the subspecialties of virology, mycology, parasitology, mycobacteriology and bacteriology that are encompassed in the disciplines of clinical, food, and environmental microbiology.
Microbiology is critical to the public health role of detecting and identifying outbreaks, emerging diseases, and biological threats. Public health laboratories serve many public health programs and provide reference and specialized testing that relate to disease control and prevention in the population. The testing services address multiple modes of transmission and include molecular methods for epidemiology and disease surveillance. In addition, public health laboratories provide many specialized tests that are not commercially available.
Notes: Multiple sources were identified as support documents for this domain (26,51,59,61,62,82). This domain is not expected to be all-inclusive of functions performed in every microbiology subspecialty in the laboratory. The General Laboratory Practice and Safety domains are companions to this domain; all are intended to be used together, though some overlap in content exists. The verb "oversees" is used extensively in the Expert level. In this context, "oversees" is a broad term that comprises the many functions related to the management of policies, processes and procedures to include creation, design, development, directing, monitoring, evaluation, and collaboration. There is an assumption for the Beginner level, especially with use of the verbs "performs" and "adheres to," that there is a degree of training and supervision still occurring that is providing needed guidance and information (e.g., on why it is critical to perform steps and processes as directed).
Chemistry Competency Guidelines
Purpose statement: The competencies in Chemistry address the knowledge, skills, and abilities needed for the qualitative and quantitative analysis of chemicals of concern to the public in biological and environmental matrices (Table 13).
Introduction: Chemistry is the science of detection, measurement, and characterization of chemicals of public health importance in samples (e.g., human and animal, food and feed, water and soil). Chemistry encompasses numerous subdisciplines in areas of both organic and inorganic testing.
Chemistry programs within public health laboratories provide a first line of defense in the rapid recognition of toxic chemical exposures and also support environmental health and epidemiological programs that investigate human exposures to chemicals in the environment. Chemistry programs also provide a wide array of specialized services related to clinical diagnostics for evaluating individual health, identification of environmental health issues, and investigation of population exposures through epidemiological programs' studies. They also aid in the response to chemical emergencies or chemical terrorism events by providing rapid and definitive testing to identify and quantify chemical agents.
Notes: Sources were identified as support documents for this domain (60,83). This domain is not expected to be all-inclusive of all chemistry-related laboratory activities. The General Laboratory Practice and Safety domains are companions to this domain; all are intended to be used together, though some overlap in content exists. The verb "oversees" is used often in the Expert level. In this context, "oversees" is a broad term that comprises the many functions related to the management of policies, processes and procedures to include creation, design, development, directing, monitoring, evaluation, and collaboration. There is an assumption for the Beginner level, especially with use of the verbs "performs" and "adheres to," that there is a degree of training and supervision still occurring that is providing needed guidance and information (e.g., on why it is critical to perform steps and processes as directed).
Bioinformatics Competency Guidelines
Purpose statement: The competencies in Bioinformatics address the knowledge, skills, and abilities needed to collect, classify, and analyze biological and biochemical information through the development and use of computer databases, algorithms, and statistical techniques (Table 14).
Introduction: Bioinformatics is the field of science that bridges the gap between biology, computer science, and information technology by merging them into a single discipline. There are three important subdisciplines within bioinformatics: the development of new algorithms and statistics with which to assess relationships among members of large data sets; the analysis and interpretation of various types of data including nucleotide and amino acid sequences, protein domains, and protein structures; and the development and implementation of tools that enable efficient access and management of different types of information.
Bioinformatics capability and capacity have become progressively more important within public health laboratories because of rapid advances in molecular technologies and laboratory techniques. As a result, the amount of data that a typical laboratory can generate has increased dramatically over the past decade. This increase in data requires new competencies for laboratory scientists to analyze and interpret large datasets, and communicate complex and complete results to audiences of varied backgrounds.
Notes: Sources were identified as support documents for this domain (84,85). This domain is intended for all laboratory scientists in addition to bioinformatics specialists.
Research Competency Guidelines
Purpose statement: The competencies in Research address the knowledge, skills, and abilities needed to conduct a systematic, hypothesis-driven investigation that includes research development, testing, and evaluation designed to advance public health knowledge, methods, and/or practice (Table 15).
Introduction: Research is a systematic investigation designed to develop or contribute to generalizable knowledge. It also includes product or method development, assessment, and evaluation. Scientific research provides information to solve new or existing problems, to reaffirm results of previous work, and to support or develop new hypotheses.
Research is critical to the public health enterprise, as communities are continually challenged with new diseases and unknown environmental public health threats. The public health laboratory community is also challenged by changes in virulence or drug susceptibility of pathogens, which impact exposure investigation and response, and the need for advanced diagnostics and analyses to support surveillance.
Notes: Multiple sources were identified as support documents for this domain (41,44,86,87). This domain is intended for use in conjunction with the General Laboratory Practice and Safety domains. The verb "oversees" is used extensively in the Expert level. In this context, "oversees" is a broad term that comprises the many functions related to the management of policies, processes and procedures to include creation, design, development, directing, monitoring, evaluation, and collaboration. This domain does not address areas of assessment and evaluation of laboratory practices, which is included in the Quality Management System and Management and Leadership domains.
Conclusion
These competency guidelines outline the knowledge, skills, and abilities needed by the PHL workforce to fulfill the responsibilities of, and demands on, the PHL system. They were developed with consideration of the diversity and complexity of PHLs. The competencies should serve as a foundation for workforce development efforts to identify and support training standards and performance expectations; develop standardized job descriptions; periodically assess individual staff and organizational capacity; and develop and implement training plans with the competencies as a guide. The competency guidelines might also be used as a framework for developing progressive job series (career ladders) for PHL workers, which has been identified as a significant barrier to worker recruitment and retention (6–8).
CDC and APHL recognize the existence of possible obstacles and challenges that might affect the implementation of these competencies. Although the uses and benefits of implementing the competencies are numerous, their adoption by PHLs might be affected by organizational and resource constraints. Acceptance and adoption of competencies in a workplace require ongoing leadership support for successful assimilation of competencies into human resources processes such as job descriptions or for integration into the curriculum of the laboratory's training and continuing education programs. Because most laboratories' human resource functions are managed by an ancillary department outside the laboratory, a collaborative effort will be needed to weave competencies into the performance management systems and hiring processes. There is also a need to educate laboratory professionals about the value of applying competencies to their daily work and a need for understanding how competencies can be used as a career ladder and management tool. On the basis of experience with the biosafety laboratory competencies published in 2011 (26), it might take several years before competencies are integrated into daily work practices (16). Successful assimilation of these competency guidelines will depend on the resources available to fully adopt and implement them and on the receptivity of laboratory professionals across the spectrum of job positions and titles. Sustained effort in these areas will be critical to strengthening the workforce and its ability to support and manage the national laboratory system.
Acknowledgments
The following persons contributed to this report: Joan Cioffi, PhD, Office of Public Health Preparedness and Response, CDC; subject matter experts from the APHL Workforce Development Committee, other PHL leadership and staff members, CDC, and practitioners from the American Society for Clinical Pathology, the American Society for Clinical Laboratory Science, the Association of Food and Drug Officials, the Association of American Feed Control Officials, the Coordinating Council on the Clinical Laboratory Workforce, the Clinical Laboratory Management Association, and the Clinical and Laboratory Standards Institute; Angela J. Beck, PhD, Matthew L. Boulton, MD, University of Michigan Center of Excellence in Public Health Workforce Studies.
References
- Association of Public Health Laboratories. Definition of a state public health laboratory system. Silver Spring, MD: Association of Public Health Laboratories; 2010. Available at http://www.aphl.org/MRC/Documents/LSS_2010Jun_Definition-of-a-State-Public-Health-Laboratory-System.pdf.
- CDC, Association of Public Health Laboratories. Core functions and capabilities of state public health laboratories: a report of the Association of Public Health Laboratories. MMWR Recomm Rep 2002;51(No. RR-14):1–8.
- Association of Public Health Laboratories. The core functions of public health laboratories. Silver Spring, MD: Association of Public Health Laboratories; 2014. Available at http://www.aphl.org/AboutAPHL/publications/Documents/APHLCoreFunctionsandCapabilities_2014.pdf.
- Association of Public Health Laboratories. Who will run America's public health labs? Educating future laboratory directors. Silver Spring, MD: Association of Public Health Laboratories; 2002. Available at http://www.aphl.org/AboutAPHL/publications/Documents/Who_Will_Run_Americas_PHL_2002.pdf.
- Beck AJ, Boulton ML. Predictors of capacity in public health, environmental, and agricultural laboratories. J Public Health Manag Pract 2014;20:654–61.
- University of Michigan Center of Excellence in Public Health Workforce Studies, Association of Public Health Laboratories. National laboratory capacity assessment, 2011. Ann Arbor, MI: University of Michigan; 2012. Available at http://www.sph.umich.edu/cephw/docs/Natl_Lab_Cap_Assess2011.pdf.
- CDC. National assessment of capacity in public health, environmental, and agricultural laboratories—United States, 2011. MMWR Morb Mortal Wkly Rep 2013;62:161–4.
- DeBoy JM, Luedtke P, Warren N, Wichman M. Basic personnel tools to help ensure a future public health and environmental laboratory workforce. Public Health Rep 2010;125(Suppl 2):96–101.
- DeBoy JM, Beck AJ, Boulton ML, Kim DH, Wichman MD, Luedtke PF. Core courses in public health laboratory science and practice: findings from 2006 and 2011 surveys. Public Health Rep 2013;128(Suppl 2):105–14.
- Wright K, Rowitz L, Merkle A, et al. Competency development in public health leadership. Am J Public Health 2000;90:1202–7.
- Czabanowska K, Smith T, Könings KD, et al. In search for a public health leadership competency framework to support leadership curriculum-a consensus study. Eur J Public Health 2014;24:850–6.
- Institute of Medicine of the National Academies. Who will keep the public healthy? Educating public health professionals for the 21st century. Washington, DC: The National Academies Press; 2003.
- Association of Schools of Public Health. Master's degree in public health core competency development project, version 2.3. Washington, DC: Association of Schools of Public Health; 2006. Available at https://www.uic.edu/sph/prepare/courses/chsc400/syllabus/mphcorecomp.pdf.
- Council on Linkages Between Academia and Public Health Practice. Core competencies for public health professionals. Washington, DC: Public Health Foundation; 2010. Available at http://www.phf.org/resourcestools/Documents/Core_Competencies_for_Public_Health_Professionals_2014June.pdf.
- CDC, Council of State and Territorial Epidemiologists. Competencies for applied epidemiologists in governmental public health agencies. Atlanta, GA: CDC, Council of State and Territorial Epidemiologists; 2008. Available at http://www.cdc.gov/appliedepicompetencies/downloads/Applied_Epi_Comps.pdf.
- Miner KR, Childers WK, Alperin M, Cioffi J, Hunt N. The MACH Model: from competencies to instruction and performance of the public health workforce. Public Health Rep 2005;120(Suppl 1):9–15.
- Birkhead GS, Koo D. Professional competencies for applied epidemiologists: a roadmap to a more effective epidemiologic workforce. J Public Health Manag Pract 2006;12:501–4.
- Koo D, Miner K. Outcome-based workforce development and education in public health. Annu Rev Public Health 2010;31:253–69, 1, 269.
- Nelson JC, Essien JDK, Loudermilk R, Cohen D. The public health competency handbook: optimizing individual & organization performance for the public's health. Atlanta, GA: Center for Public Health Practice, Emory University Rollins School of Public Health; 2002.
- CDC, University of Washington School of Public Health and Community Medicine's Center for Public Health Informatics. Competencies for public health informaticians. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/InformaticsCompetencies/downloads/PHI_Competencies.pdf.
- Columbia University School of Nursing Center for Health Policy. CDC. Bioterrorism and emergency readiness: competencies for all public health workers. New York, NY: Columbia University; 2002. Available at http://www.uic.edu/sph/prepare/courses/chsc400/resources/btcomps.pdf.
- Quad Council of Public Health Nursing Organizations. Quad Council competencies for public health nurses. Wheat Ridge, CO: Association of Community Health Nursing Educators; 2011. Available at http://www.achne.org/files/Quad%20Council/QuadCouncilCompetenciesforPublicHealthNurses.pdf.
- National Commission for Health Education Credentialing. Areas of responsibility, competencies, and sub-competencies for the health education specialists. Whitehall, PA: National Commission for Health Education Credentialing, Inc.; 2010. Available at http://www.nchec.org/credentialing/responsibilities.
- Kaml C, Weiss CC, Dezendorf P, et al. Developing a competency framework for U.S. state food and feed testing laboratory personnel. J AOAC Int 2014;97:768–72.
- Olson D, Hoeppner M, Larson S, Ehrenberg A, Leitheiser AT. Lifelong learning for public health practice education: a model curriculum for bioterrorism and emergency readiness. Public Health Rep 2008;123(Suppl 2):53–64.
- CDC, Association of Public Health Laboratories. Guidelines for biosafety laboratory competency. MMWR Suppl 2011;2011:60.
- Ridderhof JC, Moulton AD, Ned RM, et al. The laboratory efficiencies initiative: partnership for building a sustainable national public health laboratory system. Public Health Rep 2013;128(Suppl 2):20–33.
- Dreyfus SE, Dreyfus HL. A five-stage model of the mental activities involved in directed skill acquisition. Berkeley, CA: University of California–Berkeley; 1980.
- Bloom BS. Taxonomy of educational objectives, handbook I: the cognitive domain. New York, NY: David McKay Co Inc.; 1956.
- Anderson LW, Krathwohl DR, Airasian PW, et al. A taxonomy for learning, teaching, and assessing: a revision of Bloom's taxonomy of educational objectives. New York, NY: Pearson; 2000.
- CDC. Epidemic Intelligence Service. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. Available at http://www.cdc.gov/eis.
- Clinical and Laboratory Standards Institute. Standards development policies and processes. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Available at http://clsi.org/wp-content/uploads/2013/07/CLSIStandardsDevelopmentPoliciesAndProcessesFinal.pdf.
- Clinical and Laboratory Standards Institute. Quality management system: a model for laboratory services: approved guideline. 4th ed. CLSI document QMS01-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services. Laboratory requirements, 42 CFR. Chapter IV. Part 493. Available at http://www.gpo.gov/fdsys/pkg/CFR-2003-title42-vol3/xml/CFR-2003-title42-vol3-part493.xml.
- Clinical Laboratory Management Association. Body of knowledge for medical laboratory management. Chicago, IL: CLMA; 2012. Available at http://www.clma.org/p/cm/ld/fid=34.
- Clinical and Laboratory Standards Institute. Training and competence assessment: approved guideline. 3rd ed. CLSI document QMS03-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- Clinical and Laboratory Standards Institute. Laboratory personnel management. CLSI guideline QMS16—Personnel. Wayne, PA: Clinical and Laboratory Standards Institute; 2015.
- International Organization for Standardization and International Electrotechnical Commission. International Standard, ISO/IEC 17025: General requirements for the competence of testing and calibration laboratories. Geneva, Switzerland: ISO/IEC; 2005.
- Westcott RT. The certified manager of quality/organizational excellence handbook. 3rd ed. Milwaukee, WI: American Society of Quality, Quality Press; 2005.
- World Health Organization. Laboratory quality management system: handbook. Version 1.1, Lyon, France: World Health Organization; 2011. Available at http://whqlibdoc.who.int/publications/2011/9789241548274_eng.pdf?ua=1.
- Institute of Medicine. Integrity in scientific research: creating an environment that promotes responsible conduct. Washington, DC: The National Academies Press; 2002. Available at http://www.nap.edu/openbook.php?record_id=10430&page=R1.
- Jones NL. A code of ethics for the life sciences. Sci Eng Ethics 2007;13:25–43.
- Public Health Leadership Society. Principles of the ethical practice of public health. New Orleans, LA: Public Health Leadership Society; 2002. Available at http://phls.org/CMSuploads/Principles-of-the-Ethical-Practice-of-PH-Version-2.2-68496.pdf.
- Resnick DB. What is ethics in research & why is it important? Research Triangle Park, NC: NIH National Institute of Environmental Health Sciences; 2011. Available at http://www.niehs.nih.gov/research/resources/bioethics/whatis.
- Slomka J, Quill B, desVignes-Kendrick M, Lloyd LE. Professionalism and ethics in the public health curriculum. Public Health Rep 2008;123(Suppl 2):27–35.
- Bennis W. On becoming a leader. 1st ed. Cambridge, MA: Perseus Publishing; 1989.
- DeBoy J, Luedtke P, Warren N, Wichman M. Toolkit for ensuring a future workforce of qualified public health laboratory scientist-managers and directors. Chapel Hill, NC: Public Health Leadership Institute; 2006. Availableat http://www.aphl.org/aboutaphl/publications/documents/phli-aphl_project_final_report_sep302006.pdf.
- CDC. Public health preparedness capabilities: national standards for state and local planning, capability 6: information sharing. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at http://www.cdc.gov/phpr/capabilities/DSLR_capabilities_July.pdf.
- Mind Tools Ltd. Active listening. Hear what people are really saying. Wiltshire, UK: Mind Tools Ltd. Available at http://www.mindtools.com/CommSkll/ActiveListening.htm.
- National Research Council (US) Committee on Prudent Practices in the Laboratory. Laboratory security. In: Prudent practices in the laboratory: handling and management of chemical hazards, updated version. Washington, DC: National Academies Press; 2011:255–64. Available at http://www.ncbi.nlm.hih.gov/books/NBK55881.
- US Department of Health and Human Services, CDC, National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 5th ed. Washington, DC: US Department of Health and Human Services. HHS Publication No. (CDC) 21-1112; 2009. Available at http://www.cdc.gov/biosafety/publications/bmbl5/bmbl.pdf.
- World Health Organization. Biorisk management: laboratory biosecurity guidance. Geneva, Switzerland: WHO; 2006. Available at http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_EPR_2006_6.pdf.
- CDC. Emergency preparedness and response: laboratory information. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. Available at http://emergency.cdc.gov/labissues/index.asp.
- Integrated Consortium of Laboratory Networks. Integrated Consortium of Laboratory Networks web portal. Available at https://www.icln.org.
- Federal Emergency Management Agency. National preparedness goal. Washington, DC: US Department of Homeland Security, FEMA; 2015. Available at http://www.fema.gov/national-preparedness-goal.
- Federal Emergency Management Agency. National incident management system. Washington, DC: US Department of Homeland Security, FEMA; 2015. Available at https://www.fema.gov/national-incident-management-system.
- Occupational Safety and Health Administration. 29 CFR 1910.38: emergency action plans. Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2002. Available at http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9726&p_table=STANDARDS.
- American Society for Training and Development (ASTD). The ASTD training and development handbook: a guide to human resource development. 4th ed. New York, NY: The McGraw-Hill Companies; 1996.
- American Society for Microbiology. Manual of clinical microbiology. 10th ed. Washington, DC: ASM Press; 2011.
- Burtis CA, Bruns DE. Tietz fundamentals of clinical chemistry and molecular diagnostics. 7th ed. Philadelphia, PA: Elsevier Health Sciences; 2014.
- Animal and Plant Health Inspection Service, CDC. Select agent requirements, 42 CFR part 73, 7 CFR part 331 and 9 CFR part 121. Available at http://www.selectagents.gov/Regulations.html.
- CDC. Guidelines for safe work practices in human and animal medical diagnostic laboratories. MMWR Surveill Summ 2012;61(No. Suppl 1).
- Clinical and Laboratory Standards Institute. Protection of laboratory workers from occupationally acquired infections: approved guideline. 4th ed. CLSI document M29-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- Fleming DO, Hunt D. Biological safety: principles and practices. 4th ed. Washington, DC: ASM Press; 2006.
- Heymann DL. Control of communicable diseases manual. 19th ed. Washington, DC: American Public Health Association; 2008.
- International Air Transport Association. Dangerous goods regulations. 56th edition requirements. IATA; 2015. Available at http://www.iata.org/whatwedo/cargo/dgr/pages/download.aspx.
- International Organization for Standardization. Medical laboratories: particular requirements for quality and competence, second edition (ISO 15189); 2007. Available at http://www.iso.org/iso/catalogue_detail?csnumber=42641.
- National Research Council of the National Academies. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press; 2011. Available at http://oacu.od.nih.gov/regs/guide/guide.pdf.
- US Department of Labor. Toxic and hazardous substances: hazard communication (OSHA 29 CFR 1910.1200 [Final Rule]). Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2013. Available at http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10099.
- US Department of Labor. Toxic and hazardous substances: bloodborne pathogens (OSHA 20 CFR 1910.1030). Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2012. Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10051&p_table=STANDARDS.
- US Department of Labor. Toxic and hazardous substances: occupational exposure to hazardous chemicals in laboratories (OSHA 29 CFR 1910.1450). Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2012. Available at http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10106.
- US Department of Labor. Personal protective equipment: general requirements (OSHA 20 CFR 1910.132). Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2011. Available at http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9777.
- US Department of Labor. Laboratory safety guidance. Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2011. Available at http://www.osha.gov/Publications/laboratory/OSHA3404laboratory-safety-guidance.pdf.
- US Department of Labor. OSHA recordkeeping handbook. Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 2005. Available at https://www.osha.gov/Publications/recordkeeping/OSHA_3245_REVISED.pdf.
- Thacker SB, Berkelman RL. History of public health surveillance. In: Halperin W, Baker EL, Monson RR, eds. Public health surveillance. New York, NY: Van Norstrand Reinhold; 1992.
- Dicker RC, Coronado F, Koo D, Parrish RG. Principles of epidemiology in public health practice. 3rd ed. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. Available at http://www.cdc.gov/ophss/csels/dsepd/SS1978/SS1978.pdf.
- World Health Organization, Department of Communicable Disease Surveillance and Response. WHO recommended surveillance standards. 2nd ed (WHO/CDS/CSR/ISR/99.2). Geneva, Switzerland: World Health Organization. Available at http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdf.
- Association of Public Health Laboratories (APHL)/CDC. Informatics self-assessment tool for public health laboratories. Silver Spring, MD: APHL; 2013. Available at http://www.aphl.org/aphlprograms/lss/Laboratory-Efficiencies-Initiative/Pages/Informatics.aspx.
- Association of Public Health Laboratories (APHL) and the Public Health Informatics Institute. Requirements for public health laboratory information management systems. Silver Spring, MD: APHL; 2003. Available at http://www.aphl.org/Documents/Global_docs/reqs_for_PHLIMS.pdf.
- Association of Public Health Laboratories Informatics Committee. The brave new world of consolidated and shared IT services: a guide for laboratories. Silver Spring, MD: Association of Public Health Laboratories; 2011. Available at http://www.aphl.org/AboutAPHL/publications/Documents/COM_2011_ITConsolidatedandSharedServices.pdf.
- O'Carroll PW; Public Health Informatics Competencies Working Group. Informatics competencies for public health professionals. Seattle, WA: Northwest Center for Public Health Practice, University of Washington School of Public Health and Community Medicine; 2002. Available at http://www.nwcphp.org/docs/phi/comps/phi_print.pdf.
- Murray PR. Manual of clinical microbiology. 6th ed. Washington, DC: ASM Press; 1995.
- National Research Council (US) Committee on Prudent Practices in the Laboratory. Prudent practices in the laboratory: handling and management of chemical hazards, updated version. Washington, DC: National Academies Press; 2011. Available at http://www.ncbi.nlm.nih.gov/books/NBK55878.
- National Center for Biotechnology Information, US National Library of Medicine. Welcome to NCBI. Bethesda, MD: National Library of Medicine; 2009. Available at http://www.ncbi.nlm.nih.gov.
- Welch L, Lewitter F, Schwartz R, et al. Bioinformatics curriculum guidelines: toward a definition of core competencies. PLoS Comput Biol 2014;10:e1003496.
- National Postdoctoral Association. The NPA postdoctoral core competencies toolkit. New York, NY: National Postdoctoral Association; 2009. Available at http://www.nationalpostdoc.org/competencies.
- Steneck NH/CDC Office of Research Integrity. Introduction to the responsible conduct of research. Washington, DC: US Department of Health and Human Services; 2007. Available at http://ori.hhs.gov/sites/default/files/rcrintro.pdf.
* A list of all of the members of the various committees, teams, and workgroups appears beginning on page 93 of this report.
|
August 2012: The CDC/Association of Public Health Laboratories (APHL) Steering Committee for the Public Health Laboratory (PHL) Competencies Project was established. October 2012: A Project Planning Workgroup comprising CDC, APHL, and PHL representatives met to define the scope and structure of the competencies. December 2012: The Development Workgroup conducted a review of published literature and resources, including competency sets for non-PHL audiences. Outlines were crafted for use as a template for each domain. January–April 2013: Eleven Domain Teams developed draft competencies for 14 domains using the expertise of 90 subject matter experts from CDC, APHL, state and local PHLs, academic laboratories, clinical laboratories, the US Department of Agriculture, and other entities. Each Domain Team held regular, facilitated conference calls to develop and refine their competencies. April–November 2013: A Synthesis Workgroup assessed domain gaps and redundancies and harmonized language across domains. November 2013–January 2014: The competencies were vetted by volunteer representatives from external organizations and key stakeholders including PHL representatives, clinical laboratory representatives, APHL, and CDC. December 2013: The Adjudication Process Workgroup, comprising APHL, CDC, and PHL representatives, established the process for the adjudication of reviewer comments by the Domain Teams. January–March 2014: Domain Teams addressed comments received during the vetting period via conference calls. The Ethics domain was crafted as a separate domain, resulting in 15 total domains. April 2014: The Harmonization of Domains Workgroup reviewed major changes made by the Domain Teams to the draft competencies in response to reviewer comments and resolved remaining redundancies and inconsistencies in approach and language among the competency domains. April–July 2014: Small teams from the Harmonization of Domains Workgroup finalized the competency domains. October 2014: The CDC/APHL Steering Committee reviewed the competency guidelines. February 2015: CDC reviewed and approved the final guidelines. |
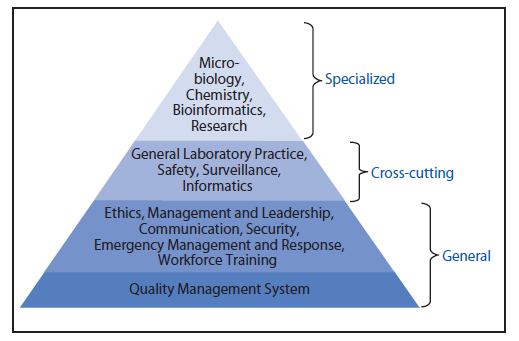
* Teams of subject matter experts develop general, cross-cutting technical, and specialized competencies, with a quality management system as the foundation of every activity.
Alternate Text: This figure is a triangle showing a schematic of competency domains for public health laboratory professionals. Teams of subject matter experts developed general, cross-cutting technical, and specialized competencies, with a quality management system as the foundation of every activity. The base of the triangle comprises general competencies: Quality Management, followed in a separate tier by Ethics, Management and Leadership, Emergency Management and Response, and Workforce Training. The middle section of the triangle comprises cross-cutting technical competences: General Laboratory Practice, Safety, Surveillance, and Informatics. The apex of the triangle comprises specialized competencies: Microbiology, Chemistry, Bioinformatics, and Research.
|
TABLE 1. (Continued) Public health laboratory competency guidelines: Quality Management System (QMS) domain |
||||
|---|---|---|---|---|
|
QMS 2.00. Customer focus: ensures that customer needs, expectations, and requirements* are consistently met |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 2.01. Customer satisfaction |
Recognizes the laboratory's internal and external customers |
Responds to internal and external customer inquiries and feedback |
Analyzes feedback and satisfaction data from internal and external customers |
Oversees the system for measuring customer and user satisfaction |
|
QMS 2.02. Customer services |
Describes the customer services provided by the laboratory to meet customer needs, expectations, and requirements |
Adheres to roles and responsibilities in meeting customer needs, expectations, and requirements |
Develops procedures to address customer needs, expectations, and requirements |
Oversees the policies, processes, and procedures for providing customer services that meet customer needs, expectations, and requirements |
|
QMS 3.00. Facilities and safety: ensures that the laboratory's physical environment, maintenance, and safety programs* meet applicable requirements |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 3.01. Workplace safety |
Participates in required workplace training regarding safety and maintenance of the physical environment |
Manages work area to ensure staff compliance with safety requirements |
Develops site-specific workplace safety policies and procedures |
Oversees the policies, processes, and procedures to develop, review, and maintain a safety plan that meets requirements |
|
QMS 3.02. Facilities |
Describes the laboratory design, escape routes, and workplace accommodations |
Monitors environmental controls* for good laboratory practice and testing capabilities |
Ensures security and containment of staff, samples,* laboratory supplies, and laboratory equipment* |
Directs the process and planning for facility design, modification, and renovation |
|
QMS 3.03. Waste management |
Describes waste management policies, processes, and procedures |
Applies waste management policies, processes, and procedures to activities |
Trains staff on the waste management plan,* including recycling and disposal methods |
Ensures the waste management plan follows regulatory requirements |
|
QMS 3.04. Emergency management and response |
Explains own role in emergency event mitigation, preparedness, response, and recovery |
Trains staff in the emergency management plan for emergency event mitigation, preparedness, response, and recovery |
Provides input on emergency management and response policies, processes, and procedures |
Establishes emergency management and response policies, processes, and procedures |
|
QMS 4.00. Personnel: ensures recruitment and retention of a qualified, well-trained, and competent workforce |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 4.01. Staff qualification process |
Describes education, training, and skills required for job performance |
Describes process required to verify staff qualification and competency |
Ensures each position has the required competencies, education, training, skills, experience, and where applicable, certification,* and licensure* |
Designs a process to determine required competencies, education, training, skills, experience and where applicable, certification and licensure for each job title |
|
QMS 4.02. Orientation and end-of-employment |
Participates in orientation and end-of-employment processes |
Ensures orientation and end-of-employment processes are carried out for each staff person |
Facilitates orientation and end-of-employment processes |
Designs an orientation and end-of-employment program |
|
QMS 4.03. Training |
Participates in required training |
Ensures that training and evaluation are carried out for assigned duties |
Facilitates training and evaluation processes |
Oversees the policies, processes, and procedures for the training program |
|
TABLE 1. (Continued) Public health laboratory competency guidelines: Quality Management System (QMS) domain |
||||
|---|---|---|---|---|
|
QMS 4.00. Personnel: ensures recruitment and retention of a qualified, well-trained, and competent workforce |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 4.04. Competence assessment plan |
Describes competence assessment plans |
Participates in the development of an individualized competence assessment plan |
Evaluates individual competence assessment plans |
Oversees a competence assessment plan for the organization |
|
QMS 4.05. Professional development plan |
Participates in initial training related to assigned work position |
Participates in continuing education and professional development opportunities |
Manages the plan to provide professional development opportunities to all levels of staff |
Designs a plan to provide professional development opportunities to all levels of staff |
|
QMS 4.06. Performance evaluation process |
Participates in the initial performance evaluation process |
Participates in the ongoing performance evaluation process |
Manages the performance evaluation process |
Establishes a process for periodic performance evaluation |
|
QMS 4.07. Recruitment, retention, and succession plans |
Describes the recruitment and retention plan |
Participates in recruitment and retention planning |
Manages the recruitment, retention, and succession plans |
Develops recruitment, retention, and succession plans to maintain a qualified workforce |
|
QMS 5.00. Purchasing and inventory: ensures that requirements for supplies and services are consistently met |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 5.01. Procurement process |
Describes laboratory procurement process for current work area |
Participates in laboratory procurement process |
Manages laboratory procurement process |
Ensures that laboratory procurement incorporates organizational rules and collaboration with purchasing authorities |
|
QMS 5.02. Inventory processes |
Describes the inventory management processes used in current work area |
Follows established inventory management processes, including receipt and inspection processes |
Develops inventory processes for laboratory supplies, reagents, and verification* of performance |
Oversees inventory management plan |
|
QMS 5.03. Evaluation process |
Describes the process to evaluate and provide feedback to suppliers |
Executes the process to evaluate and provide feedback to suppliers, consultants, and contractors |
Develops a process to evaluate the satisfaction with services and products from suppliers, consultants, and contractors |
Oversees a quality improvement plan for purchasing and inventory |
|
QMS 6.00. Laboratory equipment: ensures that laboratory equipment selection, installation, use, maintenance, and troubleshooting meet performance standards |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 6.01. Acquisition and decommissioning |
Describes the policies, processes, and procedures for equipment acquisition and decommissioning |
Provides input on the processes and procedures for equipment acquisition and decommissioning |
Develops the processes and procedures for equipment acquisition and decommissioning |
Oversees the policies, processes, and procedures for equipment acquisition and decommissioning |
|
QMS 6.02. Equipment qualification plan* |
Describes processes and procedures for equipment installation qualification, operational qualification, and performance qualification |
Performs equipment installation, operational, and performance qualification procedures |
Implements the equipment qualification plan |
Oversees the policies, processes, and procedures regarding the equipment qualification plan |
|
TABLE 1. (Continued) Public health laboratory competency guidelines: Quality Management System (QMS) domain |
||||
|---|---|---|---|---|
|
QMS 6.00. Laboratory equipment: ensures that laboratory equipment selection, installation, use, maintenance, and troubleshooting meet performance standards |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 6.03. Maintenance process |
Describes processes and procedures for the maintenance, troubleshooting, and service and repair of equipment |
Performs procedures for the maintenance, troubleshooting, and service and repair of equipment |
Develops the processes for equipment maintenance, troubleshooting, and service and repair |
Oversees the policies, processes, and procedures for equipment maintenance, troubleshooting, service, and repair |
|
QMS 6.04. Instrument and equipment calibration |
Describes calibration of instruments and equipment |
Performs calibration of instruments and equipment |
Establishes calibration processes and procedures for instruments and equipment |
Oversees calibration policies, processes, and procedures for instruments and equipment |
|
QMS 7.00. Process management:* ensures that operational processes meet organizational requirements |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 7.01. Workflow† processes |
Describes workflow processes related to job position and activities |
Applies workflow processes according to laboratory policies, processes, and procedures |
Develops evaluation, modification, and design of workflow processes and procedures |
Oversees the policies, processes and procedures for evaluating and modifying current workflow processes and for developing new workflow processes |
|
QMS 7.02. Process control |
Describes how processes are controlled in work area |
Participates in process control procedures |
Develops the process control plan |
Oversees the process control plan |
|
QMS 7.03. Method validation* and performance verification processes |
Describes method validation and performance verification processes |
Performs procedures for method validation and performance verification |
Develops method validation and performance verification processes and procedures |
Oversees the policies, processes, and procedures for validation of new or modified tests or materials and for verification of existing tests or materials |
|
QMS 8.00. Documents* and records:* ensures that there is an effective system to control and manage documents and records |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 8.01. Document management system |
Describes how the laboratory controls and manages documents |
Applies the policies, processes, and procedures for controlling and managing documents |
Develops the policies, processes, and procedures for controlling and managing documents |
Oversees the document management system to ensure staff compliance with internal policies, external regulations, and accreditation* requirements |
|
QMS 8.02. Records management system |
Describes how the laboratory controls and manages records |
Applies the policies, processes, and procedures for controlling and managing records |
Develops the policies, processes, and procedures for controlling and managing records |
Oversees the record management system to ensure staff compliance with internal policies, external regulations, and accreditation requirements |
|
TABLE 1. (Continued) Public health laboratory competency guidelines: Quality Management System (QMS) domain |
||||
|---|---|---|---|---|
|
QMS 9.00. Information* management: ensures the confidentiality,* security, and integrity of generated and disseminated information |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 9.01. Confidentiality |
Describes the policies, processes, and procedures for maintaining confidentiality of laboratory information |
Complies with policies, processes, and procedures for maintaining confidentiality of internally and externally derived information |
Develops policies, processes, and procedures for maintaining confidentiality of internally and externally derived information |
Oversees the policies, processes, and procedures for ensuring confidentiality of information and staff compliance with regulations and guidelines |
|
QMS 9.02. Security |
Describes the policies, processes, and procedures related to securing information related to assigned job tasks |
Complies with policies, processes, and procedures for securing information |
Develops policies, processes, and procedures to ensure information is secure |
Oversees the policies, processes, and procedures for securing information, including audits to meet regulations and guidelines |
|
QMS 9.03. Information integrity |
Describes the policies, processes, and procedures for ensuring integrity of information |
Complies with policies, processes, and procedures to ensure the integrity of information |
Develops processes and procedures to ensure the integrity of information |
Oversees the policies, processes, and procedures to ensure the integrity of information |
|
QMS 10.00. Nonconforming event* management: ensures that processes are in place for detecting and managing nonconforming events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 10.01. Management of nonconforming events (NCEs) |
Recognizes NCEs |
Responds to NCEs |
Investigates NCEs, including the creation of a corrective action plan |
Oversees the policies, processes, and procedures related to NCEs |
|
QMS 10.02. Documentation of NCEs |
Describes the policies, processes, and procedures to record and report NCEs |
Reports discovered NCEs |
Performs analysis of records and reports of NCEs to identify trends |
Oversees the policies, processes, and procedures to document NCEs and report NCE information to senior management and external entities |
|
QMS 10.03. Investigation and root cause analysis* |
Participates in NCE investigations and root cause analyses |
Leads the process of investigating NCEs and performing root cause analyses |
Assesses NCE investigations and root cause analyses to improve processes |
Oversees the policies, processes, and procedures for investigating NCEs and performing root cause analyses |
|
QMS 10.04. Notifications of recalls and technical bulletins |
Describes the policies, processes, and procedures to address product recalls and technical bulletin notifications |
Responds to product recalls and technical bulletin notifications |
Develops processes and procedures to address product recalls and technical bulletin notifications |
Oversees the policies, processes, and procedures to address product recalls and technical bulletin notifications |
|
QMS 11.00. Assessments: ensures that processes are in place to perform internal audits* and external assessments* |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 11.01. Quality assessment* plan |
Adheres to the quality assessment plan |
Ensures the application of the quality assessment plan to laboratory operations |
Develops the quality assessment plan |
Oversees a comprehensive quality assessment plan |
|
QMS 11.02. External assessments |
Participates in external assessment activities |
Performs external assessment procedures |
Develops the processes and procedures to select, enroll, and participate in external assessments |
Oversees the policies, processes, and procedures related to external assessment |
|
QMS 11.03. Internal audits |
Participates in internal audits |
Performs internal audit procedures |
Develops the processes and procedures for internal audits |
Oversees the policies, processes, and procedures related to internal audits |
|
TABLE 1. (Continued) Public health laboratory competency guidelines: Quality Management System (QMS) domain |
||||
|---|---|---|---|---|
|
QMS 11.00. Assessments: ensures that processes are in place to perform internal audits* and external assessments* |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 11.04. Quality indicators* |
Describes the elements of pre-examination,* examination,* and postexamination* quality indicators |
Employs pre-examination, examination, and postexamination quality indicators |
Develops processes and procedures for determining pre-examination, examination, and postexamination quality indicators |
Oversees the policies, processes, and procedures related to developing and assessing quality indicators |
|
QMS 11.05. Quality indicator data collection and analysis |
Describes the policies, processes, and procedures related to collecting and analyzing quality indicator data |
Complies with policies, processes, and procedures related to collecting and analyzing quality indicator data |
Develops the processes and procedures for collecting and analyzing quality indicator data |
Oversees the policies, processes, and procedures related to the collection and analysis of quality indicator data |
|
QMS 12.00. Continual improvement: ensures mechanisms for continuous quality improvement |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
QMS 12.01. Continuous Quality Improvement (CQI)* program |
Describes the policies, processes, and procedures related to the CQI program |
Implements changes identified through the CQI program |
Develops the processes and procedures of the CQI program |
Oversees the policies, processes, and procedures related to the quality improvement program |
|
QMS 12.02. CQI activities |
Participates in CQI activities |
Follows CQI processes and procedures for troubleshooting and documenting required CQI activities |
Documents staff compliance with CQI activities that support the CQI monitoring, evaluation, and review processes |
Oversees the policies, processes, and procedures related to CQI activities |
|
QMS 12.03. Corrective action* process |
Describes the policies, processes, and procedures related to corrective action |
Implements the processes and procedures related to corrective action |
Develops corrective action processes and procedures to address quality improvement |
Oversees the policies, processes, and procedures related to corrective action |
|
QMS 12.04. Preventive action* |
Describes the policies, processes, and procedures related to preventive action |
Implements the processes and procedures related to preventive action |
Develops the processes and procedures related to preventive action |
Oversees the policies, processes, and procedures related to preventive action |
|
QMS 12.05. Change management* |
Describes the process to change laboratory policies, processes, and procedures |
Participates in the process and procedures related to change management |
Implements the change management process, including communication of changes made to established policies, processes, and procedures |
Oversees activities related to policy, process, and procedural change management, including evaluation of impact on organizational processes and services |
|
* This term is defined in Appendix B. † Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures. |
||||
|
TABLE 2. Public health laboratory competency guidelines: Ethics domain |
||||
|---|---|---|---|---|
|
ETH 1.00. Professional code of conduct: adheres to policies* and principles governing professional ethics and rules of conduct when working in a public health laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
ETH 1.01. Personal integrity |
Aligns personal integrity with organizational culture |
Exemplifies integrity in interactions and activities |
Coaches staff in behaviors that exemplify integrity |
Creates a culture where integrity is the foundation for all interactions and activities |
|
ETH 1.02. General ethical practices* |
Applies ethical principles and professional rules of conduct to the workplace |
Serves as a role model of ethical behavior by consistently conforming to the highest ethical standards and practices |
Ensures staff compliance with policies and procedures related to ethical principles and professional rules of conduct |
Oversees the policies, processes,* and procedures* related to ethical principles and professional rules of conduct |
|
ETH 1.03. Stewardship of resources |
Acts as a good steward of public funds and resources |
Identifies methods to improve stewardship of resources |
Ensures that the use of public funds and resources meet the policies for stewardship |
Oversees the policies, processes, and procedures to ensure the environment supports excellence in the stewardship of resources |
|
ETH 2.00. Scientific code of conduct: adheres to policies and principles governing scientific ethics* and rules of conduct when working in a public health laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
ETH 2.01. Scientific integrity* |
Verifies scientific integrity of test results and findings |
Instructs others in policies, processes, and procedures regarding scientific integrity of test results and findings |
Ensures staff compliance with policies and procedures regarding scientific integrity of all results and findings |
Oversees the policies, processes and procedures to ensure practices are consistent with guidelines on scientific integrity |
|
ETH 2.02. Scientific ethics |
Applies scientific ethics and rules of conduct to the workplace |
Serves as a role model of scientific ethical behavior and rules of conduct by consistently conforming to the highest scientific standards and practices |
Ensures staff compliance with policies and procedures related to scientific ethics and rules of conduct |
Oversees the policies, processes, and procedures related to scientific ethics and rules of conduct |
|
* This term is defined in Appendix B. |
||||
|
TABLE 3. (Continued) Public health laboratory competency guidelines: Management and Leadership domain |
||||
|---|---|---|---|---|
|
MLD 1.00. General management: ensures sound management of laboratory operations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 1.06. Analytical and operational services |
Describes the processes for introducing improved analytical and operational services |
Participates in improvement of analytical and operational services |
Manages the improvement of analytical and operational services |
Oversees funding and stakeholder relationships needed to implement and improve analytical and operational laboratory services |
|
MLD 1.07. Quality* testing and services |
Describes quality principles |
Explains the importance of providing quality laboratory testing and services |
Evaluates the quality of services for continued quality improvement |
Oversees the policies, processes, and procedures to ensure the quality of laboratory testing and services |
|
MLD 1.08. Customer* service |
Supports customer service needs |
Evaluates customer service satisfaction and trends |
Identifies strategies and activities to improve customer service |
Oversees the policies, processes, and procedures to ensure the laboratory maintains a customer focus |
|
MLD 1.09. Project management* |
Uses basic project management concepts* and tools |
Trains staff on project management techniques |
Directs the management of projects at the programmatic level |
Oversees project management across the laboratory |
|
MLD 1.10. Program effectiveness |
Contributes data for program effectiveness monitoring and evaluation |
Analyzes data to assist in the monitoring and evaluation of program effectiveness |
Directs activities to monitor and evaluate the effectiveness of laboratory programs |
Oversees the policies, processes, and procedures regarding the measurement, analysis, and improvement of program effectiveness |
|
MLD 2.00. Policy development: ensures the development, implementation, and review of internal policies |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 2.01. Internal policy development |
Describes concepts pertinent to developing internal policies |
Compiles important issues, lists of stakeholders, and various options and solutions for internal policy development |
Prepares internal policies based on evaluation of short- and long-term consequences of potential policies |
Oversees the internal policy development process |
|
MLD 2.02. Internal policy implementation |
Complies with documented internal policies and guidelines |
Implements internal policies for a laboratory program |
Ensures staff compliance with laboratory-wide policies |
Oversees internal policy implementation and staff compliance |
|
MLD 2.03. Internal policy review |
Reviews internal policies for revisions and updates |
Develops amendments or updates to internal policies |
Evaluates internal policies |
Oversees the review process for internal policies |
|
MLD 3.00. Financial management: ensures sound financial management |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 3.01. Budgets |
Complies with budgetary guidelines |
Monitors staff compliance to the budget |
Reconciles budget, expenditures, and income |
Oversees budgets, including development and staff compliance with agency and legislative mandates |
|
MLD 3.02. Revenue and income |
Describes revenue sources associated with individual activities |
Tracks revenue and income for a laboratory program |
Manages revenue and income for the laboratory |
Ensures that necessary revenue and income is secured |
|
MLD 3.03. Expenditures |
Adheres to guidelines and limits for expenditures |
Tracks expenditures for a laboratory program |
Ensures staff compliance with guidelines and limits for expenditures |
Oversees the policies, process, and procedures related to the cost of operations |
|
TABLE 3. (Continued) Public health laboratory competency guidelines: Management and Leadership domain |
||||
|---|---|---|---|---|
|
MLD 3.00. Financial management: ensures sound financial management |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 3.04. Financial management process |
Uses designated financial management tools |
Integrates financial management tools for a laboratory program |
Optimizes the financial management system for the laboratory |
Oversees the policies, processes, and procedures related to financial management and fiduciary responsibility |
|
MLD 3.05. Resource management |
Uses workplace resources efficiently |
Optimizes use of laboratory program resources |
Manages resources for the laboratory |
Oversees the policies, processes, and procedures related to resource management |
|
MLD 4.00. Human resource management: ensures effective management of human resources |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 4.01. Human resource policies |
Complies with human resource rules and requirements* |
Enforces human resource requirements and policies |
Manages human resource requirements and policies |
Oversees the policies, processes, and procedures related to the human resource system |
|
MLD 4.02. Equal Employment Opportunity (EEO)* process |
Describes the EEO process |
Promotes EEO requirements and policies |
Manages EEO requirements and policies |
Oversees the policies, processes, and procedures related to EEO in the human resource system |
|
MLD 4.03. Position descriptions |
Provides input into job description content |
Evaluates position descriptions for congruency with job functions |
Manages the development and implementation processes for position descriptions |
Oversees a system of position description development and implementation to maximize staff competence and meet operational requirements |
|
MLD 4.04. Staff licensure* |
Describes the basic education, skills and certifications* for laboratory staff licensure requirements |
Maintains a process to ensure that staff meet licensure requirements |
Performs ongoing gap analysis to address staff licensure requirements |
Ensures staff compliance with licensure laws and regulations |
|
MLD 4.05. Competency assessment |
Participates in competency assessments |
Manages the competency assessment program |
Evaluates the competency assessment program |
Ensures that staff competence levels are commensurate with job functions |
|
MLD 4.06. Performance feedback |
Participates in communications with peers and supervisors regarding performance |
Integrates performance feedback into work routines |
Monitors staff progress on agreed-upon performance parameters |
Ensures that a system is in place that values honest and open communication about performance |
|
MLD 4.07. Performance appraisal process |
Describes how the formal performance appraisal process impacts laboratory operations |
Administers performance appraisals |
Evaluates effectiveness of the performance appraisal process in improving laboratory productivity and practice |
Oversees the continuous improvement of the performance management system |
|
MLD 4.08. Conflict resolution |
Identifies the need for management intervention in conflict resolution |
Resolves conflicts in a fair and equitable manner |
Integrates mechanisms to support cooperation and manage conflict across the laboratory |
Oversees the policies, processes, and procedures to ensure the fair and equitable resolution of conflicts |
|
TABLE 3. (Continued) Public health laboratory competency guidelines: Management and Leadership domain |
||||
|---|---|---|---|---|
|
MLD 4.00. Human resource management: ensures effective management of human resources |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 4.09. Progressive discipline |
Describes the steps of the progressive discipline process |
Administers progressive discipline |
Analyzes the use of progressive discipline within the laboratory |
Ensures that the progressive discipline system is used to mitigate disciplinary issues |
|
MLD 4.10. Professional development |
Participates in professional development activities |
Recommends professional development activities |
Assesses professional development gaps to ensure and support staff competency development |
Oversees the policies, processes, and procedures to encourage and address professional development |
|
MLD 4.11. Staff advancement |
Explains the policies and procedures related to staff advancement |
Recommends staff for advancement |
Develops criteria for staff advancement |
Ensures that staff function in roles commensurate with experience, skill set, and proficiency |
|
MLD 4.12. Succession planning |
Recognizes the importance of succession planning |
Implements staff development plans that align with the laboratory's succession plan |
Develops succession plans that consider current and future needs |
Oversees the policies, processes, and procedures related to the creation and implementation of succession plans |
|
MLD 5.00. Leadership: models leadership behavior |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 5.01. Ethical practices* and professional code of conduct |
Applies ethical principles and a professional code of conduct to the workplace |
Serves as a role model of ethical and professional behavior by consistently conforming to the highest standards and practices |
Ensures staff compliance with the policies and procedures related to ethical practices and a professional code of conduct |
Fosters the policies, processes, and procedures related to ethical practices and a professional code of conduct |
|
MLD 5.02. Communication |
Employs active communication skills |
Communicates information and feedback to colleagues and management staff |
Ensures the open and frequent exchange of communication between laboratory staff |
Fosters a culture of open and frequent communication |
|
MLD 5.03. Teamwork and collaboration |
Describes the value of collaboration in the workplace |
Collaborates with team members within a laboratory program |
Leads cross-functional teams to accomplish projects |
Creates a workplace environment that encourages teamwork and collaboration |
|
MLD 5.04. Diversity* culture |
Explains the value of having a diverse workforce |
Promotes a diverse workforce |
Develops programs that support a culture of diversity |
Fosters a culture where diversity is valued |
|
MLD 5.05. Staff engagement* |
Participates in activities to support the laboratory's goals |
Implements activities that support staff engagement |
Develops programs that support a culture of staff engagement |
Fosters a culture of staff engagement and commitment |
|
MLD 5.06. Staff recognition |
Participates in staff recognition programs |
Evaluates the effectiveness of staff recognition programs |
Develops staff recognition programs |
Fosters a culture that ensures staff recognition |
|
MLD 5.07. Coaching* and mentoring* |
Describes the benefits of coaching and mentoring |
Develops a pool of potential coaches and mentors for staff |
Establishes coaching and mentoring programs |
Fosters a culture where coaching and mentoring are deeply-rooted |
|
MLD 5.08. Critical thinking* |
Develops basic critical thinking skills |
Applies critical thinking to develop effective solutions to problems |
Leads critical thinking activities to achieve improvements in laboratory processes |
Fosters an environment that integrates critical thinking |
|
TABLE 3. (Continued) Public health laboratory competency guidelines: Management and Leadership domain |
||||
|---|---|---|---|---|
|
MLD 5.00. Leadership: models leadership behavior |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
MLD 5.09. Systems thinking* |
Describes systems thinking |
Applies systems thinking when approaching projects and problem solving |
Incorporates systems thinking into directing laboratory operations |
Fosters an environment that integrates systems thinking |
|
MLD 5.10. Strategic thinking* |
Describes strategic thinking |
Provides input into strategic thinking and decision-making processes |
Integrates strategic thinking into decisions and long-term planning regarding laboratory operations |
Fosters an environment that integrates strategic thinking |
|
MLD 5.11. Change management* |
Describes the value of change |
Implements change management initiatives within a laboratory program |
Leads the development of change management initiatives with the laboratory |
Fosters a culture that continuously assesses change opportunities for sustaining the mission |
|
MLD 5.12. Advocacy |
Educates external stakeholders on the mission, vision, and activities of the laboratory |
Collaborates with external stakeholders |
Identifies gaps in engagement with external stakeholders to support the activities of the laboratory |
Develops strategies to engage external stakeholders to accomplish the mission, vision, and activities of the laboratory |
|
MLD 5.13. External policy development |
Describes impacts of external policies related to the organization's mission |
Communicates with stakeholders to exchange policy input |
Promotes external policy development to support the organization's mission and vision |
Fosters relationships with strategic partners to secure laboratory-inclusive policies consistent with the organization's mission and vision |
|
MLD 5.14. Promotion of the health of populations |
Recognizes the need for partnerships to promote the health of populations |
Identifies potential partnerships to promote the health of populations |
Facilitates participation of key stakeholders to promote the health of populations |
Fosters partnerships with key stakeholders to promote the health of populations affected by laboratory services |
|
* This term is defined in Appendix B. |
||||
|
TABLE 4. (Continued) Public health laboratory competency guidelines: Communication domain |
||||
|---|---|---|---|---|
|
COM 3.00. Comprehension of materials: demonstrates comprehension of written documents* and directions |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 3.01. Reading comprehension |
Follows written directions |
Applies knowledge acquired from written text to situations |
Adapts concepts from written text for use in new situations |
Extrapolates information from written text to develop new ideas that enhance work processes* |
|
COM 4.00. Communication technology:* utilizes technology to communicate information to internal and external partners |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 4.01. Technology capability |
Describes the laboratory's and partner's technological capabilities |
Selects laboratory's technology options to align with partner's capabilities |
Evaluates existing and potential technology to align with partner's capabilities |
Establishes technology policies* that integrate with the partner's capabilities |
|
COM 4.02. Use of technology |
Describes employer's policies and procedures* for sharing information |
Uses designated technology for sharing information |
Manages technology policies and procedures used for sharing information |
Evaluates the effectiveness of the technology used for sharing information |
|
COM 5.00. Communication professionalism: ensures professionalism in communication with customers* and stakeholders |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 5.01. Professional attitude |
Uses tone of voice and language tailored to interactions with customers and stakeholders |
Displays professional demeanor in all situations with customers and stakeholders |
Monitors interactions with customers and stakeholders to ensure they are conducted professionally |
Establishes policies for professional customer and stakeholder interactions |
|
COM 5.02. Information exchange |
Provides information based on policies and procedures to meet the needs of customers and stakeholders |
Determines information needs through collaboration with customers and stakeholders |
Ensures that information exchange policies, processes, and procedures are followed to meet the needs of the customers and stakeholders |
Develops overarching system for exchange of information to meet the needs of customers and stakeholders |
|
COM 5.03. Information sharing opportunities |
Shares information as directed |
Selects information to share |
Develops information to share |
Creates opportunities for sharing information |
|
COM 6.00. Professional reports: prepares professional written reports and oral presentations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 6.01. Written reports |
Organizes information for written reports |
Creates drafts of written reports |
Revises written reports |
Establishes policies, processes, and procedures for written reports |
|
COM 6.02. Oral presentations |
Organizes information for oral presentations |
Creates drafts of oral presentations |
Revises oral presentations |
Establishes policies, processes, and procedures for oral presentations |
|
COM 7.00. Risk communication:* applies emergency and risk communication principles and techniques to explain information to targeted audiences |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 7.01. Risk communication plan |
Describes the risk communication process |
Adheres to the risk communication plan |
Ensures staff compliance with the risk communication plan |
Establishes policies, processes, and procedures related to the risk communication plan |
|
TABLE 4. (Continued) Public health laboratory competency guidelines: Communication domain |
||||
|---|---|---|---|---|
|
COM 7.00. Risk communication:* applies emergency and risk communication principles and techniques to explain information to targeted audiences |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 7.02. Emergency information |
Lists basic emergency information for target audience |
Identifies specific emergency information for target audience |
Develops emergency information and messages tailored to target audience |
Collaborates with agency leaders and partners to deliver emergency information and messages tailored to target audience |
|
COM 7.03. Empathetic risk communication |
Describes empathetic risk communication concepts |
Delivers empathetic messaging in high-risk situations or emergencies |
Creates empathetic messaging in high-risk situations and emergencies |
Evaluates empathetic messaging for high-risk situations and emergencies |
|
COM 8.00. Public health laboratory value: promotes the value of the public health laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 8.01. Public relations |
Describes impact of the work of the public health laboratory and system |
Coordinates opportunities for promoting the public health laboratory and system |
Identifies opportunities to promote the public health laboratory and system |
Develops opportunities to promote the public health laboratory and system |
|
COM 8.02. Communication to educate and inform |
Supports the development and distribution of communication materials about the public health laboratory |
Presents communication materials to explain the importance of the public health laboratory |
Develops communication materials to explain the importance of the public health laboratory |
Manages the policies, processes, and procedures regarding communication materials to explain the importance of the public health laboratory |
|
COM 8.03. Storytelling |
Shares approved public health laboratory stories |
Incorporates use of stories when communicating the impact of public health laboratory work |
Develops stories that convey information highlighting the impact of public health laboratory work |
Evaluates the impact of storytelling in promoting the public health laboratory |
|
COM 8.04. Marketing strategy |
Participates in marketing strategies |
Implements the marketing strategy for the public health laboratory |
Manages the marketing strategy for the public health laboratory |
Oversees the marketing strategy for the public health laboratory |
|
COM 9.00. Media relations: works with the media to provide information about public health laboratories and public health issues |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
COM 9.01. Media relations policies and strategies |
Adheres to agency media relations policies and procedures |
Identifies situations where agency media relations policies and strategies apply |
Monitors the application of agency media relations policies and strategies |
Oversees media relations policies and strategies |
|
COM 9.02. "Plain talk"* |
Describes the value of using "plain talk" |
Applies "plain talk" during public and media interactions |
Develops "plain talk" language for media and public communications |
Translates highly technical concepts using "plain talk" for media and public communications |
|
COM 9.03. Key messages |
Describes the value of using key messages |
Applies key messages during public and media interactions |
Develops key messages for media and public communications |
Oversees delivery of key messages on complicated, high-risk topics |
|
* This term is defined in Appendix B. |
||||
|
TABLE 5. (Continued) Public health laboratory competency guidelines: Security domain |
||||
|---|---|---|---|---|
|
SEC 2.00. Security plan:* ensures that the laboratory's security plan meets organizational goals, regulatory requirements, and established standards |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SEC 2.03. Security operations |
Describes the policies, processes, and procedures for maintaining security |
Monitors security operations |
Manages security operations to ensure defined protection measures are based on the degree of risk |
Oversees security operations |
|
SEC 2.04. Inventory records* related to security plans |
Completes inventory records to comply with the laboratory's security plan |
Audits inventory records |
Creates tools to manage inventory records |
Develops policies, processes, and procedures to manage inventory records |
|
SEC 2.05. Security incident* response |
Reports security incidents |
Evaluates reported security incidents |
Implements processes and procedures related to security incident response and reporting |
Develops policies, processes, and procedures for security incident response and reporting |
|
SEC 3.00. Physical security: ensures that physical security is maintained |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SEC 3.01. Physical security infrastructure* |
Describes the physical security infrastructure |
Trains staff on policies, processes, procedures, and related regulations regarding physical security infrastructure |
Solves difficult and complex physical security problems |
Directs the resolution of major conflicts in physical security policy and program objectives |
|
SEC 3.02. Physical security access controls* |
Describes physical security access control policies, processes, procedures, and systems |
Applies physical security access control procedures and systems |
Manages the implementation of physical security access control policies, processes, procedures, and systems |
Advises organizational authorities on methods for enhancing effectiveness and efficiency of physical security access control policies, processes, procedures, and systems |
|
SEC 4.00. Personnel security program:* implements a personnel security program to meet organizational goals, regulatory requirements, and established standards |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SEC 4.01. Personnel security concepts* |
Describes personnel security concepts |
Addresses problems or questions involving personnel security concepts |
Interprets personnel security concepts to adapt processes and procedures to support organizational goals |
Develops policies, processes, and procedures that incorporate personnel security concepts into personnel security program |
|
SEC 4.02. Personnel security program |
Complies with the laboratory's personnel security program |
Implements the personnel security program |
Solves difficult and complex personnel security problems |
Directs the resolution of major conflicts in personnel security policy and program objectives |
|
SEC 4.03. Investigations |
Collects information* for personnel security investigations |
Analyses actions regarding personnel security matters requiring investigation |
Determines actions to be taken on personnel security investigations |
Develops policies, processes, and procedures related to personnel security investigations |
|
TABLE 5. (Continued) Public health laboratory competency guidelines: Security domain |
||||
|---|---|---|---|---|
|
SEC 5.00. Information security:* ensures that information security meets organizational goals, regulatory requirements, and established standards |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SEC 5.01. Information security |
Describes policies, processes, and procedures related to information security |
Applies information security concepts, including principles of confidentiality, integrity, and availability (CIA)* |
Interprets information security concepts to adapt policies, processes, and procedures to support organizational goals |
Develops policies, processes, procedures, and organizational standards for information security to meet organizational goals |
|
SEC 5.02. Risk identification and prioritization |
Describes the risks associated with the laboratory's sensitive information* or technology related to the job being performed |
Identifies risks associated with the laboratory's sensitive information and technology, including the methods of control |
Implements processes and procedures for prioritizing risks associated with the laboratory's sensitive information and technology, including the methods of control |
Develops polices, processes, and procedures for identifying, prioritizing, and controlling sensitive information and technology |
|
SEC 6.00. Transportation security program: implements a transportation security plan* |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SEC 6.01. Transport security |
Complies with transport security policies, processes, and procedures |
Audits transport records |
Creates transport security procedures |
Establishes policies and processes to specify which materials need designated levels of transport security |
|
* This term is defined in Appendix B. † The process of identifying risks to organizational assets (including staff) and operations (including mission, functions, image, and reputation); includes threat and vulnerability analyses and is the fundamental tool to help select the right risk mitigation measures (e.g., engineered controls, standard policies and procedures) to achieve an acceptable level of security. |
||||
|
TABLE 6. (Continued) Public health laboratory competency guidelines: Emergency Management and Response domain |
||||
|---|---|---|---|---|
|
EMR 2.00. Preparation for emergency events: prepares for emergency events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
EMR 2.01. Preparation for emergency events |
Explains the laboratory's emergency response plan† and Continuity of Operations Plan (COOP)† |
Assists with development and implementation of the laboratory's emergency response plan and COOP |
Manages development and implementation of the emergency response plan and COOP for an individual area of responsibility |
Oversees laboratory's emergency response plan, including implementation of the COOP with external partners |
|
EMR 2.02. Incident command system (ICS)† |
Demonstrates awareness of the ICS by completing a FEMA-approved introductory course |
Completes higher-level FEMA-approved courses to explain the operation and management of the ICS |
Implements the ICS by completing high-level FEMA-approved courses and internal training courses and exercises |
Serves at a leadership level in the ICS |
|
EMR 2.03. Emergency response training |
Participates in emergency response training, exercises, and drills |
Contributes to the development of emergency response training, exercises, and drills |
Conducts emergency response training, exercises, and drills in collaboration with stakeholder agencies |
Oversees the collaboration with stakeholder agencies to sponsor and conduct training, exercises, and drills, ensuring proper resources are available |
|
EMR 2.04. Emergency notification |
Describes requirements† for notification of emergencies and other incidents according to organizational plans and policies |
Implements organizational plans and policies for notification of emergencies and other incidents |
Develops internal policies and procedures for notification of emergencies and other incidents |
Oversees the collaboration with stakeholders and agencies to develop and implement plans and policies for notification of emergencies and other incidents |
|
EMR 2.05. Identification of key partners |
Describe partners and their relationships with the institution |
Interacts with partners on staff |
Engages partners to sustain relationships and ensure effective response |
Develops new partnerships to ensure effective emergency response |
|
EMR 2.06. Execution of agreements |
Describes emergency agreements between the institution and other partners |
Updates agreements with partners to ensure emergency response capability |
Trains staff on agreements in place to ensure emergency response capability |
Negotiates agreements between partner organizations to ensure emergency response capability |
|
EMR 2.07. Emergency preparedness and response networks† |
Explains how the laboratory interacts with emergency preparedness and response networks |
Describes the plans, policies and procedures the institution has in place to prepare for and respond to a public health emergency |
Develops the organizational plans, policies and procedures to prepare for and respond to a public health emergency |
Oversees the collaboration with emergency preparedness and response networks to develop and implement plans, policies, and procedures to prepare for and respond to a public health emergency |
|
EMR 3.00. Responding to emergency events: responds to emergency events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
EMR 3.01. Situational briefing |
Participates in meetings and conference calls to receive information† on the situation |
Prepares information on the situation |
Facilitates the briefing of key stakeholders to create situational awareness |
Manages implementation of COOP measures and ICS activation |
|
EMR 3.02. ICS activation |
Performs position responsibilities as assigned |
Produces information and documentation for briefings |
Confirms staff are available with proper qualifications and capabilities |
Establishes organization's ICS structure, reporting procedures, and chain of command |
|
EMR 3.03. Emergency evacuation |
Locates emergency evacuation routes and assembly areas |
Uses emergency evacuation routes and assembly areas |
Instructs staff during evacuation |
Manages emergency evacuation and assembly |
|
TABLE 6. (Continued) Public health laboratory competency guidelines: Emergency Management and Response domain |
||||
|---|---|---|---|---|
|
EMR 3.00. Responding to emergency events: responds to emergency events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
EMR 3.04. Hazardous spill† response |
Recognizes hazardous spills or potential exposures |
Complies with procedures for responding to hazardous spills or potential exposures |
Instructs staff on proper response to hazardous spills or potential exposures |
Manages response to hazardous spills or potential exposures |
|
EMR 3.05. Emergency decontamination† |
Describes emergency decontamination and exposure prevention† policies and procedures |
Complies with emergency decontamination and exposure prevention policies and procedures |
Instructs staff on policies, processes, and procedures for emergency decontamination and exposure prevention |
Manages emergency decontamination and exposure prevention policies, processes, and procedures |
|
EMR 3.06. Surge capacity |
Describes circumstances for, and varying degrees of surge |
Adjusts workflow§ to ensure timeliness of diagnostic testing in collaboration with surge partners |
Identifies creative strategies to manage surge or overflow testing |
Implements a management system that promotes flexibility and maximizes the ability to deliver surge capacity |
|
EMR 3.07. Emergency communication plan† |
Describes the emergency communication plan and the policies and procedures for receiving and disseminating information with emergency response partners and/or public |
Complies with the emergency communication plan and the policies and procedures for receiving and disseminating information with emergency response partners and/or public |
Ensures rapid and secure communications with emergency response partners and/or public during emergencies and surge incidents |
Manages the emergency communication plan and the policies, processes, and procedures for securely receiving and disseminating information with emergency response partners and the public during emergencies and surge incidents |
|
EMR 4.00. Recovering from emergency events: recovers from emergency events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
EMR 4.01. Short-term recovery |
Follows established plans to resume normal operations after an emergency event |
Implements plans to resume normal operations after an emergency event |
Determines whether staff and facilities are able to return to normal operations after an emergency event |
Manages re-opening and/or continuation of routine services |
|
EMR 4.02. After Action Review (AAR)† |
Describes the AAR process |
Participates in AAR process |
Assists with developing the final AAR |
Oversees the AAR process |
|
EMR 4.03. Long-term recovery |
Lists improvements to laboratory plans based on the AAR |
Identifies improvements to laboratory plans and operations based on the AAR |
Implements improvements to laboratory plans and operations based on the AAR |
Develops recommendations to improve laboratory plans and operations based on the AAR along with internal and external partners and stakeholders |
|
EMR 4.04. Financial considerations |
Lists costs relative to response and recovery activities |
Explains costs relative to response and recovery activities |
Determines financial and staff resources required to facilitate laboratory's response and recovery |
Verifies financial and staff resources are in place to facilitate laboratory's response and recovery |
|
EMR 4.05. Legal and regulatory issues |
Describes legal and regulatory requirements for managing emergency events |
Implements legal and regulatory requirements for managing emergency events |
Assesses staff compliance with legal and regulatory requirements related to the managing of emergency events |
Oversees staff compliance with legal and regulatory requirements related to the managing of emergency events |
|
* The process of identifying risks to organizational assets (including staff) and operations (including mission, functions, image, and reputation); includes threat and vulnerability analyses and is the fundamental tool to help select the right risk mitigation measures (e.g., engineered controls, standard policies and procedures) to achieve an acceptable level of security. † This term is defined in Appendix B. § Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures. |
||||
|
TABLE 7. (Continued) Public health laboratory competency guidelines: Workforce Training domain |
||||
|---|---|---|---|---|
|
WFT 2.00. Training design: designs training |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
WFT 2.04. Cultural awareness |
Explains cultural awareness and its importance as it relates to developing training activities |
Applies cultural awareness principles when designing training activities |
Evaluates the incorporation of cultural awareness principles into training activities |
Ensures incorporation of cultural awareness principles into training activities |
|
WFT 2.05. Instructional materials preparation |
Assembles instructional materials |
Prepares instructional materials for existing programs |
Develops instructional materials for new programs that are aligned with the type of training activity and modality |
Incorporates industry-wide instructional materials into the training program |
|
WFT 2.06. Training materials application |
Uses pre-existing training materials to design simple training |
Integrates multiple types of training materials into training design |
Develops training materials from industry resources |
Evaluates training materials from industry resources |
|
WFT 2.07. Training exercises |
Delivers exercises within a training session |
Integrates individual training lessons, including experiential exercises |
Evaluates training exercises |
Designs integrated training exercises with partners |
|
WFT 2.08. Formative assessment* |
Explains formative assessment |
Employs formative assessments |
Creates formative assessments |
Evaluates effectiveness of formative assessments |
|
WFT 2.09. Continuing education |
Explains the requirements of the continuing education provider |
Follows continuing education provider requirements when conducting training |
Selects continuing education provider for new learning activities |
Oversees the continuing education provider process |
|
WFT 3.00. Delivery set-up: manages the logistics of set-up for training delivery |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
WFT 3.01. Equipment preparation for training delivery |
Operates equipment needed to deliver training |
Troubleshoots training equipment |
Ensures that equipment capability aligns with the training requirements |
Oversees processes for upgrading training equipment |
|
WFT 3.02. eLearning* |
Tests eLearning courses |
Creates supporting materials or content for storyboards |
Develops storyboards for new courses using subject matter expert materials |
Ensures that eLearning delivery systems are available |
|
WFT 3.03. Learning environment* |
Provides support for learning environment processes |
Manages processes of the learning environment |
Develops processes to manage the learning environment |
Oversees the processes for the learning environment |
|
WFT 4.00. Training delivery: applies principles of learning to training implementation and delivery |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
WFT 4.01. Learning preferences and styles |
Explains presentation materials for different learning preferences and styles |
Differentiates presentation materials to address learning preferences and styles |
Develops presentation materials to address learning preferences and styles |
Creates strategies to address learning preferences and styles |
|
WFT 4.02. Presentation engagement |
Explains the most effective presentation tools and techniques |
Uses the most effective presentation tools and techniques |
Ensures implementation of the most effective presentation tools and techniques |
Oversees presentation engagement strategies |
|
TABLE 7. (Continued) Public health laboratory competency guidelines: Workforce Training domain |
||||
|---|---|---|---|---|
|
WFT 5.00. Training evaluation: evaluates learner knowledge and skill development |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
WFT 5.01. Training evaluation process* |
Lists the steps the organization undertakes for training program evaluation |
Carries out the evaluation steps for standard training evaluations for routine courses |
Develops training evaluation tools* for a new activity |
Oversees the evaluation of the training process for the organization |
|
WFT 5.02. Delivery of the evaluation |
Contributes to development of a training assessment rubric* to ensure training outcomes are met |
Implements the training assessment rubric to ensure training outcomes are met |
Creates a training assessment rubric to ensure training outcomes are met |
Evaluates the training assessment rubric to ensure training outcomes are met |
|
WFT 5.03. Training reports* |
Gathers data as directed for summative training reports |
Compiles tracking data into summative training reports |
Develops summative training reporting tools |
Interprets summative data from reports for delivery to stakeholders |
|
WFT 5.04. Training activity effectiveness |
Shares training activity observations with supervisor |
Assesses participants' achievement of training objectives |
Recommends improvements based on evaluation data from training assessment tools |
Implements improvements to the professional development activities of the laboratory |
|
WFT 5.05. Continuous improvement of the training program |
Participates in continuous improvement activities |
Identifies activities leading to the continuous improvement of a training plan |
Facilitates activities leading to the continuous improvement of a training plan |
Develops a training program improvement plan based on program evaluation |
|
WFT 6.00. Marketing: markets training opportunities |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
WFT 6.01. Marketing |
Participates in the marketing of training |
Composes content for marketing materials |
Develops organizational marketing plan for training |
Manages the training marketing plan |
|
* This term is defined in Appendix B. |
||||
|
TABLE 8. (Continued) Public health laboratory competency guidelines: General Laboratory Practice domain |
||||
|---|---|---|---|---|
|
GEN 1.00. General technical and laboratory practice knowledge: demonstrates general knowledge and skills related to the scientific and technical components of laboratory testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 1.07. Documentation |
Documents actions and results using established paper or electronic systems |
Instructs others in use of paper or electronic methods or systems for documentation |
Ensures utilization of established paper and electronic documentation methods or systems |
Oversees the policies, processes, and procedures for the creation and use of paper and electronic methods or systems for documentation |
|
GEN 1.08. Stewardship of resources |
Acts as a good steward of public funds and resources |
Identifies methods to improve stewardship of resources |
Ensures that the use of public funds and resources meet the policies for stewardship |
Oversees the policies, processes, and procedures to ensure the environment supports effective stewardship of resources |
|
GEN 1.09. Scientific ethics* |
Applies scientific ethics and rules of conduct to the workplace |
Serves as a role model, consistently conforming to the highest scientific standards and practices |
Ensures staff compliance with the policies and procedures related to scientific ethics and rules of conduct |
Oversees the policies, processes, and procedures related to scientific ethics and rules of conduct |
|
GEN 2.00. Reagent use and storage: adheres to policies and principles regarding the use and storage of laboratory reagents and supplies |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 2.01. Use and storage of reagents and supplies |
Adheres to policies, processes, and procedures for use and storage of reagents and supplies |
Instructs staff in use and storage of reagents and supplies |
Ensures staff compliance with policies, processes, and procedures for use and storage of reagents and supplies |
Oversees the use and storage of reagents and supplies |
|
GEN 2.02. Reagent preparation |
Adheres to policies, processes, and procedures for preparing reagents |
Instructs staff in preparing reagents |
Ensures staff compliance with policies, processes, and procedures for reagent preparation |
Oversees the policies, processes, and procedures for reagent preparation |
|
GEN 3.00. Equipment use: adheres to policies and principles regarding the use, maintenance, and calibration of laboratory equipment |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 3.01. Equipment operation |
Adheres to policies, processes, and procedures for operating laboratory equipment |
Instructs staff in the operation of laboratory equipment |
Ensures staff compliance with policies, processes, and procedures for the operation of laboratory equipment |
Oversees the policies, processes, and procedures for the operation of laboratory equipment |
|
GEN 3.02. Equipment maintenance |
Performs routine system checks and maintenance |
Instructs staff in procedures to ensure equipment function |
Determines need for repair or replacement of laboratory equipment |
Oversees the policies, processes, and procedures for the maintenance, repair, and replacement of laboratory equipment |
|
GEN 3.03. Instrument and equipment calibration |
Performs calibration of routine instruments and equipment |
Performs calibration of complex instruments and equipment |
Develops processes and procedures for calibration of instruments and equipment |
Oversees the policies, processes, and procedures for calibration of instruments and equipment |
|
GEN 3.04. Preventive maintenance and calibration records* |
Documents maintenance and calibration activities |
Inspects preventive maintenance and calibrations records for completeness |
Evaluates the preventive maintenance and calibration records |
Oversees the preventive maintenance and calibration program |
|
TABLE 8. (Continued) Public health laboratory competency guidelines: General Laboratory Practice domain |
||||
|---|---|---|---|---|
|
GEN 4.00. Pre-examination:* performs steps in the pre-examination phase of testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 4.01. Sample management* |
Follows policies, processes, and procedures for the management of samples* |
Instructs others in policies, processes, and procedures for sample management |
Monitors staff compliance with established sample management policies, processes, and procedures |
Oversees sample management policies, processes, and procedures |
|
GEN 5.00. Examination:* performs steps in the examination phase of testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 5.01. Sample analyses |
Performs sample analyses |
Instructs staff in sample analyses |
Ensures staff compliance with policies, processes, and procedures for sample analyses |
Oversees the policies, processes, and procedures related to sample analyses |
|
GEN 5.02.Testing workflow† |
Adheres to policies, processes, and procedures for testing workflow |
Instructs staff in policies, processes, and procedures regarding testing workflow |
Ensures staff compliance in following established testing workflow |
Oversees the policies, processes and procedures that optimize and improve testing workflow |
|
GEN 5.03. Quality control (QC)* analysis |
Performs QC activities |
Interprets QC data prior to reporting results |
Examines QC data over time to establish QC ranges and limits |
Oversees the policies, processes, and procedures related to QC activities, including staff compliance |
|
GEN 6.00. Postexamination:* performs steps in the postexamination phase of testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 6.01. QC evaluation |
Assembles QC data for evaluation |
Evaluates QC data for a given data reporting period |
Ensures staff compliance with established policies, processes, and procedures for QC evaluation activities |
Oversees the policies, processes, and procedures related to QC evaluation activities |
|
GEN 6.02. Test analysis and results interpretation |
Assembles test data for review and action |
Analyzes test data |
Interprets complex or ambiguous results |
Oversees the policies, processes, procedures, and algorithms related to data analysis and results interpretation |
|
GEN 6.03. Results reporting and data release |
Adheres to policies, processes, and procedures related to reporting and release of examination results and notifiable results* |
Instructs staff in the policies, processes, and procedures related to reporting and release of examination results and notifiable results |
Ensures staff compliance with policies, processes, and procedures related to reporting and release of examination results and notifiable results |
Oversees the policies, processes, and procedures related to reporting and release of examination results and notifiable results to partners |
|
GEN 6.04. Turnaround time (TAT) |
Performs laboratory testing and reporting within specified or expected TAT |
Monitors TAT performance |
Identifies process efficiencies to improve TAT |
Oversees the policies, processes, and procedures related to TAT |
|
GEN 6.05. Quality assurance (QA)* |
Explains the differences between QA and QC |
Collects data for reporting on QA indicators and processes |
Evaluates QA indicator data |
Oversees the policies, processes, and procedures related to QA |
|
TABLE 8. (Continued) Public health laboratory competency guidelines: General Laboratory Practice domain |
||||
|---|---|---|---|---|
|
GEN 7.00. Regulatory compliance: complies with regulations and guidelines governing laboratory testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
GEN 7.01. Regulatory compliance |
Complies with regulatory requirements* and guidelines related to laboratory testing |
Instructs staff on regulatory requirements and guidelines related to laboratory testing |
Ensures staff compliance with regulatory requirements and guidelines related to laboratory testing |
Oversees the policies, processes, and procedures regarding regulatory requirements and guidelines related to laboratory testing |
|
GEN 7.02. Proficiency testing* (PT) and alternative assessment* |
Performs PT and alternative assessment |
Reviews PT and alternative assessment results |
Monitors to ensure the PT and alternative assessment program meets regulatory requirements |
Oversees the policies, processes, and procedures related to PT and alternative assessments |
|
GEN 7.03. Proficiency testing (PT) and alternative assessment reporting |
Reports PT and alternative assessment |
Reviews submissions of PT and alternative assessment results |
Ensures staff compliance with reporting of PT and alternative assessment results |
Oversees the policies, processes, and procedures related to PT and alternative assessment reporting |
|
GEN 7.04. Method validation* and performance verification* |
Participates in performance of method validation and performance verification |
Compiles results of method validation and performance verification |
Evaluates method validation and performance verification results |
Oversees the policies, processes, and procedures related to method validation and performance verification |
|
GEN 7.05. Protected information* |
Complies with policies, processes, and procedures regarding protected information |
Instructs staff in policies, processes, and procedures regarding protected information |
Ensures staff compliance with policies, processes, and procedures regarding protected information |
Oversees that organizational policies, processes, and procedures related to protected information align with laws and regulatory requirements and guidelines |
|
* This term is defined in Appendix B. † Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures. |
||||
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: potential hazards |
||||
|
SPH 2.00. Biological materials:* works safely with biological materials in the laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SPH 2.01. Biological materials used in the laboratory |
Lists the biological materials in the laboratory |
Distinguishes biohazardous materials* from nonbiohazardous materials in the laboratory |
Manages the inventory of biological materials to ensure it is complete and updated |
Establishes the policies,* processes,* and procedures* for implementing a biological materials inventory system |
|
SPH 2.02. Hazards associated with the biological materials handled in the laboratory |
Describes hazards associated with the biological materials handled in the laboratory |
Recognizes hazards associated with new biological materials used in laboratory procedures |
Assesses staff knowledge of the hazards associated with biological materials used in laboratory procedures |
Ensures that staff are skilled in describing and recognizing hazards associated with the biological materials used in the laboratory |
|
SPH 2.03. Control measures to be used when working with biological materials |
Describes the control measures to be used when working with biological materials |
Implements the control measures to be used when working with biological materials |
Ensures that staff implement the established control measures when working with biological materials |
Establishes the control measures to be used when working with biological materials |
|
SPH 2.04. Work practices to be used when working with biological materials |
Describes the work practices to be used when working with biological materials |
Implements the work practices to be used when working with biological materials |
Ensures that staff implement the established work practices when working with biological materials |
Establishes the work practices to be used when working with biological materials |
|
SPH 2.05. Hazards associated with laboratory procedures |
Describes hazards associated with the laboratory procedures employed |
Trains staff in the hazards associated with the laboratory procedures employed |
Manages the hazards associated with laboratory procedures |
Ensures that staff are capable of recognizing, training, and managing the hazards associated with laboratory procedures |
|
SPH 3.00. Research animals:* works safely with research animals |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SPH 3.01. Hazards associated with research animals |
Describes hazards associated with working with research animals |
Identifies hazards associated with the particular species of animals used in the laboratory's research |
Assesses staff knowledge of the hazards associated with the particular species of animals used in the laboratory's research |
Ensures that staff are skilled in describing and recognizing hazards associated with the research animals used in the laboratory's research |
|
SPH 3.02. Route of exposure* to infectious agents in the animal care setting |
Describes possible route(s) of exposure to infectious agents in relation to animal procedures |
Identifies the possible route(s) of exposure to infectious agents in relation to the animal procedures used in the laboratory or animal facility |
Assesses staff knowledge of the hazards associated with the animal procedures used in the laboratory or animal facility |
Evaluates possible route(s) of exposure to infectious agents in relation to the animal procedures used in the laboratory and animal facilities |
|
SPH 3.03. Control measures to be used when working with research animals |
Describes control measures to be used when working with research animals |
Implements control measures to be used when working with research animals |
Ensures that staff implement the established control measures when working with research animals |
Establishes the control measures to be used when working with research animals |
|
SPH 3.04. Work practices to be used when working with research animals |
Describes work practices to be used when working with research animals |
Implements work practices to be used when working with research animals |
Ensures that staff implement the established work practices when working with research animals |
Establishes the work practices to be used when working with research animals |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: potential hazards |
||||
|
SPH 4.00. Chemical materials:* works safely with chemical materials in the laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SPH 4.01. Chemicals used in the laboratory |
Identifies chemicals used in the laboratory |
Distinguishes hazardous chemicals* from nonhazardous chemicals in the laboratory |
Manages the chemical inventory to ensure it is complete and updated |
Establishes the policies, processes, and procedures for implementing a chemical inventory system |
|
SPH 4.02. Hazards associated with chemicals used in the laboratory |
Describes hazards associated with chemicals used in the laboratory |
Recognizes hazards associated with new chemicals used in the laboratory |
Assesses staff knowledge of the hazards associated with chemicals used in the laboratory |
Ensures that staff are skilled in describing and recognizing hazards associated with chemicals used in the laboratory |
|
SPH 4.03. Control measures to be used when working with chemicals in the laboratory |
Describes control measures to be used when working with chemicals as documented in the laboratory's Chemical Hygiene Plan* |
Implements established control measures when working with chemicals according to the laboratory's Chemical Hygiene Plan |
Ensures that staff implement the established control measures when working with chemicals in compliance with the laboratory's Chemical Hygiene Plan |
Establishes the laboratory's Chemical Hygiene Plan, including specific control measures to be used when working with chemicals |
|
SPH 4.04. Work practices to be used when working with chemicals in the laboratory |
Describes the work practices to be used when working with chemicals as documented in the laboratory's Chemical Hygiene Plan |
Implements established work practices when working with chemicals according to the laboratory's Chemical Hygiene Plan |
Ensures that staff implement established work practices when working with chemicals in compliance with the laboratory's Chemical Hygiene Plan |
Establishes the laboratory's Chemical Hygiene Plan, including specific work practices to be used when working with chemicals |
|
SPH 5.00. Radiological materials:* works safely with radiological materials in the laboratory |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SPH 5.01. Radiological materials used in the laboratory |
Lists the radiological materials used in the laboratory |
Describes the characteristics of the radiological materials used in the laboratory |
Manages the inventory of radiological materials to ensure it is complete and updated |
Establishes the policies, processes, and procedures for implementing a radiological materials inventory system |
|
SPH 5.02. Hazards associated with the use of radiological materials |
Describes the hazards associated with radiological materials used in the laboratory |
Recognizes hazards associated with new radiological materials used in the laboratory |
Assesses staff knowledge of the hazards associated with radiological materials used in the laboratory |
Ensures that staff are skilled in describing and recognizing the hazards associated with radiological materials used in the laboratory |
|
SPH 5.03. Control measures to be used when working with radiological materials |
Recognizes control measures to be used when working with radiological materials in the laboratory |
Implements control measures to be used when working with radiological materials in the laboratory |
Ensures that staff implement established control measures when working with radiological materials in the laboratory |
Establishes, in collaboration with radiation safety staff, the control measures to be used when working with radiological materials in the laboratory |
|
SPH 5.04. Work practices to be used when working with radiological materials |
Describes work practices to be used when working with radiological materials in the laboratory |
Implements work practices to be used when working with radiological materials in the laboratory |
Ensures that staff implement established work practices when working with radiological materials in the laboratory |
Establishes, in collaboration with radiation safety staff, the work practices to be used when working with radiological materials in the laboratory |
|
SPH 5.05. Radiation monitoring devices* |
Describes monitoring devices for the radiological materials used in the laboratory |
Demonstrates operation and use of monitoring devices for the radiological materials used in the laboratory |
Ensures the operation and use by staff of radiation monitoring devices |
Evaluates use and suitability of monitoring devices for the radiological materials used in the laboratory |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: hazard control* |
||||
|
SHC 1.00. Engineering controls:* implements intervention strategies to control hazards by systematically minimizing, isolating, or removing hazards from the workplace |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 1.01. Engineering controls |
Describes engineering controls |
Employs engineering controls to eliminate or reduce targeted laboratory hazards |
Develops standard operating procedures (SOPs)* and work instructions that incorporate engineering controls |
Ensures the implementation of policies, processes, and procedures related to engineering control design, creation, and use |
|
SHC 1.02. Training on engineering controls |
Completes required training before using engineering controls |
Trains staff on engineering controls |
Develops required training for engineering controls |
Ensures that training is adequate and appropriate for the engineering controls used in the laboratory |
|
SHC 1.03. Function verification* and maintenance of engineering controls |
Describes function verification, maintenance, and troubleshooting procedures for engineering controls |
Performs function verification, maintenance, and troubleshooting processes and procedures for engineering controls |
Manages the procedures for function verification, maintenance, and troubleshooting for engineering controls |
Develops policies, processes, and procedures to ensure function verification, maintenance, and troubleshooting for engineering controls |
|
SHC 1.04. Malfunction of engineering controls |
Recognizes when engineering controls are compromised, malfunctioning, or nonfunctioning, and the resulting reporting requirements* |
Implements procedures to address and report when engineering controls are compromised, malfunctioning, or nonfunctioning |
Manages processes and procedures for addressing and reporting situations in which engineering controls are compromised, malfunctioning, or nonfunctioning |
Develops policies, processes, and procedures for remediation and reporting of engineering control malfunctions to ensure minimal exposure and release of targeted hazards |
|
SHC 2.00. Safe work practices: designs work practices and procedures to minimize exposure to hazards and to adhere to regulatory requirements |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 2.01. Good housekeeping procedures* |
Describes good housekeeping procedures |
Practices good housekeeping procedures |
Develops processes and procedures related to the establishment and maintenance of good housekeeping |
Oversees the implementation of policies, processes, and procedures related to good housekeeping |
|
SHC 2.02. Personal hygiene procedures* |
Describes personal hygiene procedures |
Complies with personal hygiene procedures |
Develops personal hygiene procedures |
Ensures staff adherence to personal hygiene policies, processes, and procedures |
|
SHC 2.03. Safety practices and procedures |
Describes proper work practices and procedures |
Uses proper work practices and procedures |
Develops proper work practices and procedures |
Ensures staff knowledge and use of proper work practices and procedures |
|
SHC 2.04. Work schedules |
Describes how adherence to own scheduled work activities and tasks minimizes exposure |
Monitors staff adherence to established work schedules and assigned tasks |
Implements procedures to ensure scheduling of work activities and/or workers' tasks minimize staff exposure levels |
Designs processes and procedures to ensure scheduling of work activities and/or workers' tasks minimize staff exposure levels |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: hazard control* |
||||
|
SHC 3.00. Personal Protective Equipment (PPE):* employs the selection, use, and care of personal protective equipment while being continually mindful of its limitations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 3.01. PPE selection |
Describes appropriate PPE and its limitations for jobs assigned |
Selects appropriate PPE for jobs assigned |
Develops procedures for the appropriate selection of PPE |
Ensures staff knowledge of procedures for the appropriate selection of PPE |
|
SHC 3.02. PPE use |
Describes specific PPE and its limitations for use with each laboratory procedure |
Uses specific PPE for each laboratory procedure |
Determines procedures for use of specific PPE |
Ensures staff compliance with procedures for use of specific PPE |
|
SHC 3.03. PPE inspection |
Describes pre-and postinspection procedures for PPE |
Implements pre-and postinspection procedures for PPE |
Develops pre-and postinspection procedures for PPE |
Ensures staff knowledge of pre-and postinspection procedures for PPE |
|
SHC 4.00. Systems to track hazards: establishes a system to detect and to control or eliminate the underlying causes of hazards or exposures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 4.01. Hazard reporting, tracking, and investigation |
Describes the procedures for reporting hazardous conditions |
Implements procedures for reporting and tracking all hazards |
Develops procedures to report, track and investigate hazards in their workspace |
Ensures staff compliance with reporting, tracking, and investigating hazards in the workplace |
|
SHC 5.00. Preventive maintenance: conducts regular maintenance to ensure effective functioning of laboratory equipment* and to extend the life of equipment |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 5.01. Planned maintenance |
Performs required preventive maintenance functions |
Complies with processes and procedures to ensure equipment continues to function effectively |
Implements the processes and procedures to ensure equipment continues to function effectively |
Develops the policies, processes, and procedures to ensure equipment continues to function effectively |
|
SHC 5.02. Corrective maintenance |
Describes troubleshooting methods to determine whether equipment is malfunctioning and the cause |
Complies with processes and procedures to ensure equipment repairs |
Implements the processes and procedures to ensure equipment repairs |
Develops the policies, processes, and procedures to ensure repairs are conducted in accordance with organizational safety and health procedures |
|
SHC 6.00. Decontamination* and laboratory waste management: establishes a laboratory waste management plan* that adheres to federal, state, and local regulations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 6.01. Decontamination of laboratory waste |
Describes procedures for spill cleanup and decontamination of laboratory waste |
Implements procedures for spill cleanup and decontamination of laboratory waste |
Manages procedures for spill cleanup and decontamination of laboratory waste |
Develops policies, processes, and procedures for spill cleanup and decontamination of laboratory waste |
|
SHC 6.02. Segregated waste categorization* and handling |
Describes procedures for laboratory waste categorization and handling |
Implements procedures for laboratory waste categorization and handling |
Manages procedures for laboratory waste categorization and handling |
Ensures staff compliance with laboratory waste categorization and handling policies, processes, and procedures |
|
SHC 6.03. Treatment and disposal |
Describes procedures for disposal and treatment of laboratory waste |
Implements procedures for disposal and treatment of laboratory waste |
Manages procedures for disposal and treatment of laboratory waste |
Ensures staff compliance with policies, processes, and procedures for disposal and treatment of laboratory waste |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: hazard control* |
||||
|
SHC 6.00. Decontamination* and laboratory waste management: establishes a laboratory waste management plan* that adheres to federal, state, and local regulations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SHC 6.04. Waste reduction |
Describes procedures for laboratory waste reduction |
Implements procedures for laboratory waste reduction |
Manages procedures for laboratory waste reduction |
Ensures staff compliance with policies, processes, and procedures for laboratory waste reduction |
|
SHC 6.05. Regulated waste* access |
Describes procedures for preventing public access to regulated waste |
Implements procedures for preventing public access to regulated waste |
Manages procedures for preventing public access to regulated waste |
Ensures staff compliance with policies, processes, and procedures for preventing public access to regulated waste |
|
SHC 6.06. Waste management issues and problems |
Describes procedures for reporting and responding to issues or problems regarding laboratory waste management |
Implements procedures for reporting and responding to issues or problems regarding laboratory waste management |
Develops procedures to ensure that issues or problems regarding laboratory waste management are reported and addressed |
Ensures staff compliance with policies, processes, and procedures to address laboratory waste management issues or problems |
|
SHC 6.07. Monitoring and evaluation |
Describes procedures for monitoring the laboratory waste management plan |
Implements procedures for monitoring the laboratory waste management plan |
Develops procedures for monitoring the laboratory waste management plan |
Ensures staff compliance with policies, processes, and procedures for monitoring the laboratory waste management plan |
|
Safety subdomain: administrative controls* |
||||
|
SAC 1.00. Safety program* management: manages the laboratory safety program |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SAC 1.01. Safety program |
Complies with the safety program requirements for the jobs performed |
Ensures staff compliance with safety program requirements |
Implements the safety program and related training programs |
Ensures that comprehensive safety policies, processes, and procedures are developed as part of the safety program |
|
SAC 1.02. Program audits |
Participates in audits of the safety program |
Conducts audits of the safety program |
Designs safety program audits |
Evaluates the safety program audit results to identify problem areas |
|
SAC 1.03. Safety inspections |
Explains the importance of safety inspections |
Participates in safety inspections |
Conducts safety inspections |
Ensures staff compliance with safety inspections |
|
SAC 1.04. Program evaluation |
Provides feedback on the safety program |
Collects data relating to the effectiveness of the safety program |
Prepares evaluation reports for the safety program |
Designs evaluation reports for the safety program |
|
SAC 2.00. Guideline and regulation compliance: ensures staff compliance with guidelines and regulations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SAC 2.01. Regulatory requirements and guidelines |
Describes current regulatory requirements and guidelines governing the safe performance of laboratory procedures |
Complies with current regulatory requirements and guidelines governing the safe performance of laboratory procedures |
Instructs staff on current regulatory requirements and guidelines governing the safe performance of laboratory procedures |
Ensures staff compliance with current regulatory requirements and guidelines governing the safe performance of laboratory procedures |
|
SAC 2.02. Institutional safety committees* |
Describes institutional safety committees |
Complies with institutional safety committee requirements |
Participates in a leadership role on institutional safety committees |
Ensures staff compliance with institutional safety committee requirements |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: administrative controls* |
||||
|
SAC 3.00. Risk management: manages risks through systematic practices to evaluate, minimize, or eliminate them |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SAC 3.01. Risk assessment† |
Describes the risk assessment process |
Implements control measures identified in risk assessments |
Manages the risk assessment process |
Oversees the policies, processes, and procedures related to risk assessment to ensure controls are appropriate for activities, agents and materials used in laboratory |
|
SAC 3.02. Incident* reporting |
Reports any incidents, including near-misses |
Reviews reports of incidents to identify root causes and problems |
Conducts routine monitoring of staff compliance regarding incident reporting |
Designs policies, processes, and procedures for reporting and performing root-cause analyses of incidents |
|
SAC 4.00. Occupational health and medical surveillance: complies with occupational health and medical surveillance policies |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SAC 4.01. Vaccination program |
Describes the organization's vaccination program |
Identifies staff eligible to participate in the vaccination program |
Monitors staff compliance with the vaccination program |
Designs the vaccination program based on exposure risks and regulatory requirements |
|
SAC 4.02. Medical surveillance program* |
Describes the organization's medical surveillance program |
Identifies staff eligible to participate in the medical surveillance program |
Monitors staff compliance with the medical surveillance program |
Designs the medical surveillance program based on risks encountered and regulatory requirements |
|
SAC 4.03. Exposure monitoring* |
Describes exposure monitoring procedures |
Complies with exposure monitoring procedures |
Ensures staff compliance with exposure monitoring policies and processes |
Develops the exposure monitoring policies and processes based on risks encountered and regulatory requirements |
|
SAC 4.04. Occupational incidents |
Identifies the process to obtain medical services after an occupational incident |
Complies with organizational requirements and healthcare provider treatment plans pertaining to an occupational incident |
Prepares summary of occupational incidents |
Develops occupational incident response plan* including regular review and revisions following an occupational incident |
|
Safety subdomain: communication and training |
||||
|
SCT 1.00. Hazard communication:* promotes safety through effective hazard communication |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SCT 1.01. Safety signage* |
Describes safety signage and documentation as well as how signage is used to convey information |
Adheres to information and directives in safety signage and documents* |
Evaluates safety signage and document placement and usage |
Ensures staff compliance with safety signage and documents |
|
SCT 1.02. Safety communication tools |
Describes a variety of communication tools and techniques promoting the work practices employed in own area of responsibility |
Employs a variety of communication tools and techniques promoting the work practices employed in own area of responsibility |
Implements a variety of communication tools and techniques for the promotion of safe work practices |
Ensures a variety of communication tools and techniques promoting work practices are employed in their area of responsibility |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: communication and training |
||||
|
SCT 1.00. Hazard communication:* promotes safety through effective hazard communication |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SCT 1.03. Labeling |
Describes labeling of samples* and containers |
Adheres to procedures for labeling of samples and containers |
Implements procedures to ensure staff compliance with regulatory requirements for labeling of samples and containers |
Ensures staff compliance with regulatory requirements for labeling of samples and containers |
|
SCT 1.04. Signals and alarms |
Recognizes signals and alarms in areas assigned |
Explains signals and alarms in the laboratory facility |
Assesses staff knowledge of signals and alarms |
Ensures the implementation of all signals and alarms |
|
SCT 2.00. Safety training: ensures that safety training needs are identified and training solutions are implemented to meet performance and productivity goals |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SCT 2.01. Safety training |
Complies with requirements to obtain safety training |
Provides training on the work practices and techniques required for staff to safely perform their job duties |
Assesses safety training needs and the impact of safety training |
Ensures development and implementation of safety training for all staff |
|
SCT 2.02. Training documentation |
Describes requirements for documenting safety training |
Adheres to procedures for recording safety training of staff |
Implements procedures for documenting staff safety training |
Develops policies, processes, and procedures for documentation and verification of staff training records* |
|
Safety subdomain: documents and records |
||||
|
SDR 1.00. Documents and record keeping: ensures staff compliance with agency quality management system (QMS)* and statutory, regulatory, accreditation,* and licensing* requirements for documentation and recordkeeping in relation to the health and safety management systems |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SDR 1.01. Safety document management |
Describes procedures for safety document management |
Adheres to procedures for safety document management |
Manages safety document management process |
Designs the safety document management system |
|
SDR 1.02. Safety document access |
Identifies any restricted or confidential safety documents |
Ensures restricted or confidential safety documents are not disclosed |
Implements processes and procedures for maintaining confidentiality* of internally and externally derived safety information |
Designs policies, processes, and procedures for document control and access that adhere to regulatory and accreditation requirements |
|
SDR 1.03. Occupational injuries and illnesses documentation |
Describes responsibilities for documenting Occupational Safety and Health Administration (OSHA)* recordable occupational injuries and illnesses |
Complies with documentation procedures for OSHA-recordable occupational injuries and illnesses |
Ensures staff compliance with reporting of OSHA-recordable occupational injuries and illnesses |
Designs policies, processes, and procedures to ensure reporting for OSHA-recordable occupational injuries and illnesses |
|
SDR 1.04. Medical surveillance documentation |
Describes responsibilities in complying with established medical surveillance recordkeeping procedures |
Complies with established medical surveillance documentation and recordkeeping procedures |
Implements processes and procedures for medical surveillance documentation |
Designs policies, processes, and procedures for medical surveillance documentation |
|
TABLE 9. (Continued) Public health laboratory competency guidelines: Safety domain |
||||
|---|---|---|---|---|
|
Safety subdomain: documents and records |
||||
|
SDR 1.00. Documents and record keeping: ensures staff compliance with agency quality management system (QMS)* and statutory, regulatory, accreditation,* and licensing* requirements for documentation and recordkeeping in relation to the health and safety management systems |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SDR 1.05. Exposure monitoring documentation |
Describes the procedures for documenting exposure monitoring |
Adheres to procedures for documenting exposure monitoring |
Implements procedures for documentation and retention of exposure monitoring information as required by regulations |
Designs policies, processes, and procedures for documentation and retention of exposure monitoring information as required by regulations |
|
SDR 1.06. Safety inspection documentation |
Describes safety inspection documentation |
Complies with procedures for safety inspection documentation |
Develops procedures for safety inspection documentation |
Designs policies, processes, and procedures for safety inspection documentation |
|
SDR 1.07. Hazardous waste documentation |
Describes procedures for documenting the handling and transport of hazardous waste |
Complies with procedures for documenting the handling and transport of hazardous waste |
Develops procedures for documenting the handling and transport of hazardous waste |
Ensures staff compliance with policies, processes, and procedures for documenting the handling and transport of hazardous waste |
|
SDR 1.08. Safety reports to staff members |
Reads safety reports |
Complies with recommendations and mandates of safety reports |
Ensures staff compliance to recommendations and mandates made in safety reports |
Designs policies, processes, and procedures ensuring staff compliance to recommendations and mandates made in safety reports |
|
* This term is defined in Appendix B. † The evaluation of the probability and consequences of exposure to a given hazard, with the intent to reduce the risk by establishing the appropriate hazard controls to be used. |
||||
|
TABLE 10. (Continued) Public health laboratory competency guidelines: Surveillance domain |
||||
|---|---|---|---|---|
|
SRV 3.00. Surveillance testing: performs surveillance testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 3.02. Surveillance testing workflow§ |
Describes test sample collection, storage, and analytical method workflows |
Employs established testing workflow and test methods for the surveillance target requirements |
Customizes testing workflow policies and procedures to the surveillance target requirements |
Directs development and modifications to surveillance testing system workflows |
|
SRV 3.03. Surveillance testing |
Tests samples for surveillance |
Participates in performance evaluation of surveillance test methods and testing capabilities |
Applies technical knowledge to develop test methods and testing capabilities used in surveillance |
Oversees the selection and creation of public health surveillance tests |
|
SRV 3.04. Outbreak* or exposure event* detection |
Describes at least one definition of an outbreak or exposure event |
Informs supervisor of potential outbreak or exposure event |
Reports potential outbreak or exposure events to key stakeholders |
Contributes to the modification of outbreak or exposure event recognition processes or definitions |
|
SRV 3.05. Sample collection for outbreak or exposure events |
Receives outbreak or exposure event samples for testing |
Ensures that outbreak or exposure event samples meet sample collection criteria |
Collaborates with key stakeholders to determine the best samples to collect |
Contributes to sample collection guidelines for outbreak or exposure event scenarios |
|
SRV 3.06. Testing for outbreak or exposure events |
Follows sample prioritization schema for testing during an outbreak or exposure event |
Ensures that outbreak or exposure event samples are prioritized according to schema |
Reports outbreak or exposure event testing results to key stakeholders |
Represents the laboratory in After Action Reviews* for outbreak or exposure events |
|
SRV 4.00. Response to critical surveillance event:* responds to critical surveillance events |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 4.01. Critical event planning |
Describes critical event response processes and procedures related to surveillance |
Applies critical event processes and procedures related to surveillance |
Evaluates plans and After Action Review findings following critical events |
Modifies the policies, processes, and procedures for critical surveillance events based on results of exercises or actual events |
|
SRV 4.02. Critical event response |
Describes own critical event response duties related to surveillance |
Manages resources required to respond to critical events |
Implements the critical event response plan |
Oversees the activation and termination of the critical event response plan |
|
SRV 4.03. Coordination of response |
Describes the need to coordinate testing priorities |
Communicates testing priorities to staff and laboratory management |
Coordinates testing activities during critical events to align with identified testing priorities |
Ensures implementation of response plans during critical events |
|
SRV 4.04. New testing capabilities |
Identifies when current testing capabilities do not exist to test an analyte or organism |
Applies technical knowledge to implement new testing capabilities |
Develops plans to address testing capabilities for a specific new organism or analyte during critical surveillance events |
Determines the overall strategy for development of new testing capabilities during critical surveillance events |
|
TABLE 10. (Continued) Public health laboratory competency guidelines: Surveillance domain |
||||
|---|---|---|---|---|
|
SRV 5.00. Information for surveillance: recognizes vital information needed for surveillance |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 5.01. Demographic information |
Enters demographic information that is necessary to carry out surveillance testing activities |
Communicates the required demographic information to submitters |
Ensures submission of crucial demographic information by submitters |
Determines demographic information fields required in informatics systems, requisition forms, and reports |
|
SRV 5.02. Sample information |
Enters sample information that is necessary to carry out surveillance testing activities |
Monitors the capture of sample information |
Ensures collection of sample information |
Determines policies, processes, and procedures for sample information collection based on jurisdictional requirements and guidelines |
|
SRV 6.00. Data analysis: analyzes data from surveillance testing systems |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 6.01. Data analysis |
Describes surveillance data analysis methods |
Analyses laboratory surveillance data |
Interprets laboratory surveillance data |
Develops standards for data analysis and for interpretation of laboratory surveillance data |
|
SRV 7.00. Data management: manages public health surveillance data using secure data management systems |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 7.01. Data collection |
Conducts data entry |
Determines validity and reliability of data collection instruments and methods |
Ensures data collection system adheres to laboratory, local, and national standards |
Coordinates modifications to data collection systems using state and national guidance and methods |
|
SRV 7.02. Data storage and retrieval |
Uses secure and stable data storage and retrieval systems |
Ensures that the design of storage and retrieval databases include the necessary variables and data dictionary |
Develops secure and stable data storage and retrieval systems, including creating new variables as necessary to support analysis of data |
Develops standards for secure and stable data storage and retrieval |
|
SRV 8.00. Recognition of significant results: recognizes significant results in surveillance data |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 8.01. Significant values and results |
Adheres to policies and procedures to verify significant results |
Reports significant results with interpretation to laboratory management and customers* |
Monitors significant results to ensure staff compliance with policies for reporting |
Develops plans with customers to identify significant results in the population or environment |
|
SRV 8.02. Trends in data |
Provides trend results to laboratory management |
Identifies trends in surveillance data |
Explains trends in surveillance data to laboratory management and customers |
Evaluates testing capabilities based on trend data to address customer needs and public health issues |
|
TABLE 10. (Continued) Public health laboratory competency guidelines: Surveillance domain |
||||
|---|---|---|---|---|
|
SRV 9.00. Partnerships: maintains partnerships to conduct surveillance |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 9.01. Multidisciplinary teamwork |
Communicates approved laboratory information to other institutions |
Implements the multi-disciplinary surveillance communication plan |
Manages the multi-disciplinary surveillance communication plan |
Develops a multi-disciplinary surveillance communication plan |
|
SRV 9.02. Education and feedback for partners |
Explains sample requirements and testing procedures |
Guides partners in selection of laboratory methods, data collection, and evaluation |
Evaluates effectiveness and efficiency of surveillance processes and procedures between laboratory and partners |
Develops surveillance policies, processes, and procedures with partners |
|
SRV 10.00. Dissemination of data: disseminates data relevant to audience |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
SRV 10.01. Presentation of surveillance and monitoring data |
Describes importance and use of oral and written communication in presenting surveillance and monitoring data |
Reports surveillance and monitoring data orally or in writing to laboratory management and epidemiologists |
Explains surveillance and monitoring data orally or in writing to external stakeholders |
Synthesizes surveillance and monitoring data orally and in writing for national and international audiences for policy decision-making purposes |
|
* This term is defined in Appendix B. † A detailed plan for conducting a scientific procedure. § Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures. |
||||
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 1.00. Laboratory test request and sample* receiving: manages sample receiving and the processing of laboratory test requests |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 1.06. Capture of auxiliary data |
Describes required identifiers and core data elements |
Verifies the routine entry of metadata and pass-through auxiliary data |
Manages problem resolution concerning entry of metadata and auxiliary data |
Designs modules to automate the entry of auxiliary data, the identification of core data elements, and the inclusion of new data elements |
|
INF 2.00. Test preparation, Laboratory Information Management System (LIMS)* processing, test results recording and verification: manages systems for electronic test preparation, LIMS processing, and test results recording and verification |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 2.01. Test preparation and receipt of samples |
Describes use of predefined electronic modules to assign samples or batches* of samples to processes in the laboratory |
Verifies the assignment of samples to individual test processes or test processing combinations |
Manages prioritization for preparation and handling of samples |
Designs systems to automate the electronic management of pre-examination* operations on samples received individually or in batch |
|
INF 2.02. Electronic test requests from submitters |
Describes how to use electronic modules to manage individual or batches of samples from submitters |
Verifies the receipt of samples and associated electronic test requests from submitters |
Ensures that valid values and test codes are properly harmonized between submitters and receiving laboratory |
Designs systems to automate the electronic management of test requests from submitters |
|
INF 2.03. LIMS tracking of testing processes and associated sample sources |
Describes how to use electronic modules to track testing processes and associated sample sources |
Verifies effectiveness of ongoing sample source tracking and submitter's monitoring efforts |
Evaluates the LIMS tracking of testing processes and associated sample sources |
Designs systems to automate and manage the tracking of testing processes and associated sample sources |
|
INF 2.04. Test results recording |
Describes electronic modules, vocabulary, and usage for specific test results |
Populates test results data using pre-existing modules |
Troubleshoots automated test results data capture utilities |
Designs analytical sequences for instrument integration and data capture utilities to automate data transfer from instruments |
|
INF 2.05. Data review |
Describes preparation of data summaries that are used for review processes |
Verifies data and results using predefined progress reports |
Evaluates the need for new tracking reports to facilitate data review |
Develops workflows and utilities to ensure that needed data are supplied |
|
INF 2.06. Data verification |
Explains test result choices that exist for specific test requests |
Verifies that the electronic transfer of quality assurance (QA)* and quality control (QC)* data* occurs to ensure that test results meet procedural requirements* and auto-assignment |
Institutes rules to ensure that laboratory programs have tools to manage data verification processes |
Designs the processes for automating data verification and associating QA and QC data with individual sample tests and batches before reporting |
|
INF 2.07. Auto-assignment of reflex* or repeat testing |
Describes the electronic processes that define auto-assignment of reflex or repeat testing |
Verifies that auto-assignment of reflex or repeat testing is performed |
Evaluates the processes for auto-assignment of reflex or repeat testing |
Develops code for design and configuration of processes for automating the assignment of reflex or repeat testing |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 3.00. Report preparation and distribution: manages test result report creation and distribution |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 3.01. Processing of data for reports |
Describes electronic modules used to print data and information* in automated reports |
Verifies analytical data, information, and reports |
Appraises solutions for data selection for nonroutine reports and for research purposes |
Constructs queries for ad hoc data searches |
|
INF 3.02. Report production |
Describes use of electronic modules to generate automated and manual reports or test results |
Selects results for reporting using standardized predefined report formats |
Evaluates report production processes |
Develops code to query and report laboratory data |
|
INF 3.03. Electronic reporting |
Sends predefined reports via electronic reporting formats |
Reports test results using predefined electronic messages that meet agreed-upon standards |
Manages the tracking and needs assessment* of electronic reporting of data |
Develops reports that contain electronic messages for test results using agreed-upon standards and vocabulary for message creation and transport |
|
INF 3.04. Management of reports |
Prints predefined reports according to policies* |
Verifies that reports adhere to submitters' data exchange format requirements for electronic results submittal and reporting |
Modifies noncomplex electronic formats to meet customer* requirements |
Develops automated processes to manage reporting of results |
|
INF 4.00. Laboratory test scheduling: manages laboratory test scheduling |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 4.01. Scheduling documents* |
Prints existing worksheets, work lists, and test scheduling documents |
Prioritizes test scheduling to resolve conflicts and turnaround time |
Develops work lists, worksheets, and test scheduling documents |
Develops processes for integration of documents to link test orders with test results |
|
INF 4.02. Management of test schedules |
Enters data to remove or restore completed test requests |
Uses specific data elements associated with process improvement to manage test schedules |
Evaluates the workflow for process improvement opportunities |
Develops codes to automate the creation of test status reports and workflows |
|
INF 4.03. Prioritization of tests |
Logs in samples according to predefined generic priorities |
Manages test requests using laboratory-specified criteria |
Organizes specific data elements associated with process improvement indicators* to prioritize test scheduling |
Develops systems to electronically generate a real-time test schedule |
|
INF 5.00. Prescheduled testing: manages prescheduled testing |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 5.01. Prescheduling |
Describes electronic modules for receipt and processing of pre-scheduled samples and kit distribution |
Manages scheduling of single or recurring test requests |
Troubleshoots electronic systems to preschedule tests and to predict and adjust workload |
Develop automated processes to manage the receipt and processing of pre-scheduled samples, recurring test requests, and kit distribution |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 6.00. Sample tracking and chain of custody:* manages the tracking of physical samples and chain of custody |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 6.01. Tracking samples at accessioning |
Uses predefined electronic modules to enter data relevant to track all steps in the sample lifecycle* |
Assigns identifiers to samples |
Evaluates automated modules that verify system operations regarding tracking |
Develops systems to determine sample tracking and location |
|
INF 6.02. Chain of custody |
Uses predefined modules to track and document custody of the sample from receipt to disposal or return to submitter |
Ensures staff compliance with chain of custody policies and procedures |
Manages electronic tracking data by validating that chain of custody is complete and documented |
Develops systems to electronically automate the communication of chain of custody data tracking to users and submitters |
|
INF 6.03. Chain of custody data elements |
Uses predefined modules to link demographic data with data on chain of custody, sample appropriateness,* sample handling, and elements of sample analyst location, time, and defined storage parameters |
Verifies that predefined modules allow creation, tracking, and maintenance of sample and aliquot hierarchy through the LIMS throughout the laboratory |
Evaluates existing and future modules for tracking data elements |
Develops systems to integrate data elements into automated chain of custody management |
|
INF 6.04. Tracking samples in analytical processes |
Uses predefined modules to track samples assigned to laboratory programs during analytical processes |
Verifies the routine tracking of aliquots, instrument sequence numbers, and work lists |
Creates work lists, worksheets, and workgroups to improve the tracking of samples |
Develops modules to incorporate factors that affect automated assignment of samples to work lists and integration of sample tracking into routine laboratory functions |
|
INF 7.00. Media, reagents, and controls: manages the manufacturing and inventory of media, reagents, and controls electronically |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 7.01. Supplies tracking |
Applies predeveloped electronic modules to order supplies and control inventory |
Verifies the tracking, management, and maintenance of inventory |
Determines the availability and cost-effectiveness of in-house manufacturing and use of supplies |
Designs code or scripts to automate activities to track, order, and manage inventory |
|
INF 7.02. Inventory production |
Performs data entry into existing electronic modules to document production of inventory |
Validates the production of inventory |
Evaluates data to improve the current and future states of inventory production |
Writes code or scripts to automate inventory control |
|
INF 7.03. Manufacturing formulations |
Accesses manufacturing formulations electronically |
Maintains the database of manufacturing formulations |
Validates manufacturing formulations and SOPs |
Develops workflows for manufacturing formulations |
|
INF 7.04. Supply orders and vendors |
Enters inventory order data into electronic order systems |
Verifies the use of codes and parameters necessary to automate electronic orders of supplies |
Manages order frequency and timetables |
Develops workflows to automate the ordering of supplies from vendors |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 8.00. Data exchange and interoperability: manages the electronic exchange of laboratory data with data partners |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 8.01. Laboratory data collection |
Explains data elements and formats necessary for data collection and exchange |
Identifies analytical data and results and the exchange of this information using predefined modules |
Develops automated interfaces to export data from instruments to electronic storage |
Configures modules to automate the export of analytical data to electronic storage |
|
INF 8.02. Electronic messaging |
Describes use of predefined modules to deliver predefined messages to partners |
Performs predefined queries of analytical data for electronic messaging |
Develops ad hoc queries for electronic messaging |
Maps electronic data to form messages consistent with client message structure, format, and vocabulary for export |
|
INF 8.03. Electronic messaging transport |
Describes predefined electronic messaging transport protocols§ |
Sends automated electronic results to partners according to established electronic messaging transport protocols |
Determines secure electronic messaging transport protocols |
Ensures the use of secure electronic messaging transport protocols |
|
INF 8.04. Message vocabulary |
Describes vocabulary necessary for data exchange |
Verifies local codes are pre-mapped to nationally accepted standard codes for test requests and test results |
Evaluates processes that automate the linking of local and national codes |
Automates the mapping of test codes and results to all standardized notifiable diseases and conditions |
|
INF 8.05. Test order creation |
Describes standard test order vocabulary |
Communicates test orders with partners using predefined modules |
Manages the automation of test orders in collaboration with partners |
Develops protocols for automated electronic test order creation |
|
INF 8.06. Test order receipt and notification |
Describes standard test order receipt and notification vocabulary |
Processes test orders received from partners using predefined modules |
Manages the automation of test order receipts and notification in collaboration with partners |
Develops protocols for electronic test order receipts and notification |
|
INF 8.07. Test results reporting |
Describes standard test results vocabulary |
Communicates test results with partners using predefined modules |
Manages the automation of test results reporting in collaboration with partners |
Develops protocols for electronic test order results reporting |
|
INF 8.08. Test results acknowledgment |
Describes test results receipt acknowledgment |
Verifies test results receipt with partners using predefined modules |
Manages the automation of test results receipts in collaboration with partners |
Develops protocols for electronic test results receipts |
|
INF 8.09. Exchange networks |
Describes exchange networks |
Performs routine data exchange using predefined modules |
Troubleshoots exchange network interfacing |
Manages the workflow and operation of exchange networks according to information exchange standards |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 9.00. Statistical analysis and surveillance: generates statistical analyses of analytical results for public health surveillance |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 9.01. Meta data and demographic data |
Enters meta data and demographic data associated with laboratory testing |
Verifies the collection and editing of meta data and demographic data using predefined modules |
Ensures the ability to capture, retrieve, and link meta data and demographic data associated with laboratory testing |
Develops modules and workflows to electronically capture, retrieve, and link meta data and demographic data associated with laboratory testing |
|
INF 9.02. Statistical analysis |
Explains how automated statistical evaluation tools link meta data and demographic data within reports |
Performs predefined queries on collected data for predefined statistical analyses to link meta data and demographic data within reports |
Develops ad hoc queries to collect extracts of data for unique statistical analyses to link meta data and demographic data within reports |
Writes code or procedure code for third-party software to automate the querying and reporting of statistical data to link meta data and demographic data within reports |
|
INF 9.03. Laboratory performance analyses |
Describes the production of laboratory performance reports |
Provides reporting and evaluation of laboratory program performance data using predefined reports |
Develops ad hoc statistical analyses to evaluate key performance indicators |
Develops workflows and automation tools to ensure the implementation of laboratory performance-based analyses |
|
INF 9.04. Spatial data |
Describes modules for entering geographic information system (GIS) data |
Uses predefined tools to integrate GIS data with laboratory testing results and with meta and demographic data |
Evaluates the availability of validated tools to integrate GIS data with laboratory testing results and with meta and demographic data |
Develops modules for LIMS-associated or third-party software to integrate GIS data with laboratory testing results and with meta and demographic data |
|
INF 10.00. Billing for laboratory services: manages billing for laboratory services |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 10.01. Billing data |
Links existing billing code(s) with associated laboratory services at time of accessioning |
Troubleshoots billing data associated with laboratory services |
Evaluates the automated capability to link billing data with laboratory services |
Develops workflows and modules to manage the collection of financial data |
|
INF 10.02. Accounts receivable* |
Enters billing data in an accounts receivable program |
Performs advanced functions of automated electronic billing and linking of laboratory services to accounts receivable systems |
Manages accounts receivable systems regarding billing of services rendered |
Develops workflows and modules to automate the integration of billing information with accounts receivable software and financial services |
|
INF 10.03. Cost of testing and other laboratory services |
Identifies accounting codes assigned for laboratory services rendered |
Verifies that correct accounting codes are consistent with the cost of services and are linked to laboratory services rendered |
Manages accounts for cost of laboratory services to individual customers |
Develops workflows and modules to track the cost of laboratory services with customers and to manage the accounts receivable system |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 10.00. Billing for laboratory services: manages billing for laboratory services |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 10.04. Integration of laboratory billing with enterprisewide billing |
Describes billing functions for multiple systems that handle billing of laboratory services |
Verifies that accounting of laboratory services is being collected from disparate systems |
Evaluates centralized functionality regarding an enterprise-wide capability to account for costs |
Develops workflows and systems to consolidate cross-enterprise billing and accounting for laboratory services |
|
INF 10.05. Budgeting* |
Describes billing modules |
Performs routine reporting of billing and revenue data for fiscal analyses |
Generates detailed budgetary summaries of billing data |
Develops systems to link billing data for budgeting and trend analysis |
|
INF 11.00. Contract* and grant* management: manages grants and contractual instruments* |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 11.01. Document management systems |
Describes centralized electronic document management systems that track and store grants, contractual instruments, and project management* materials |
Verifies the entry and timeliness of laboratory program deliverables into document management systems |
Evaluates document management systems to recommend improvements and efficiency and to meet contractual and grant obligations |
Develops enterprise-wide workflows and communications to ensure an automated and secure document management system for grants and contractual instruments |
|
INF 11.02. Contractual instruments |
Describes informatics support available to laboratory through contractual instruments |
Verifies the use of existing contractual instruments |
Manages contractual instruments |
Creates contractual instruments with partners to ensure informatics and information technology (IT) needs are captured |
|
INF 11.03. Activity tracking |
Describes informatics contractual and grant deliverables |
Tracks activities and deliverables of grants and contractual instruments using predefined electronic modules |
Evaluates the laboratory's ability to track delivery of individual informatics components related to budgetary, personnel, legal, and laboratory procedures and processes |
Develops systems to define, organize, monitor, and track the activities of grants and contractual instruments with outside parties using electronic processes |
|
INF 11.04. Enterprise-wide systems |
Describes contractual informatics instruments relevant to the enterprise |
Explains contractual instruments developed for individual laboratory programs for enterprise management |
Evaluates informatics contractual instruments with outside parties using electronic documentation processes |
Develops an enterprise-wide approach to information systems implementation |
|
INF 12.00. Training, education, and resource management: manages training, education, and information resources |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 12.01. Electronic master record* |
Accesses electronic information on staff training, education, and capabilities |
Verifies that electronic documentation of training, education, and management of educational resources is up-to-date |
Evaluates that electronic documentation of training, education, and related information meets operational requirements |
Develops modules to ensure electronic content, access, and security exist to meet the educational needs of the laboratory |
|
INF 12.02. Resource summaries |
Lists available electronic reports that summarize laboratory resources, including staff |
Verifies that electronic reports regarding laboratory resources are accurate and complete |
Evaluates the need for additional and revised reports on laboratory resources |
Develops modules to ensure electronic summary reports of laboratory resources are available |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 12.00. Training, education, and resource management: manages training, education, and information resources |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 12.03. Workforce development |
Describes informatics systems for tracking documented staff needs and knowledge gained from training opportunities |
Verifies that informatics processes remain up-to-date and demonstrate the acquired informatics capabilities of staff |
Evaluates laboratory informatics needs to ensure the existence of resources and avenues to support staff education and training |
Develops informatics programs to ensure that staff education and training is aligned with the business needs and directions for laboratory services and the professional growth of staff |
|
INF 12.04. Training activities for external partners |
Describes existing electronic documentation on trainings offered to external partners |
Verifies electronic documentation on training activities for external partners are accurate and complete |
Evaluates electronic training activities for external partners to ensure they meet current and future requirements |
Develops electronic training materials and associated documentation for external partners |
|
INF 12.05. Knowledge management (KM)* |
Describes own role(s) in supporting the collective knowledge within a laboratory program |
Verifies the participation and integration of program staff to ensure electronic KM within the organization |
Evaluates the effectiveness of electronic KM practices organizationally |
Develops a strategy for the creation, collection, and management of KM performance measures electronically |
|
INF 12.06. Lifecycle management strategy* for IT investments |
Describes laboratory IT project management resources |
Ensures the use of IT project management resources |
Evaluates staff compliance with a comprehensive lifecycle management strategy for IT investments |
Develops a comprehensive laboratory IT lifecycle management strategy |
|
INF 12.07. Informatics communication strategy |
Describes the strategy for communicating with internal and external partners regarding informatics capabilities and resourcing priorities |
Ensures the implementation of the communication strategy |
Evaluates staff compliance with the communication strategy |
Develops a strategy for communication of informatics capabilities and resourcing priorities to internal and external partners |
|
INF 13.00. Laboratory certifications,* accreditations,* and licensing:* ensures adherence to local, state, and federal certification, accreditation, and licensing requirements |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 13.01. Certification, accreditation, and licensing (CAL) compliance |
Explains applicable CAL electronic data requirements related to work area |
Verifies the implementation of predefined data processing standards and form management associated with CAL for a laboratory program |
Evaluates electronic data processing standards in order to ensure laboratory adherence to current and new CAL requirements |
Develops workflows and modules to automate laboratory adherence to data processing standards associated with CAL |
|
INF 13.02. External certification |
Enters data into predefined modules |
Verifies performance of predefined electronic systems regarding external certifications |
Evaluates current capabilities to manage external certifications and future needs |
Develops electronic modules to automate the management of external certifications |
|
INF 13.03. Privacy and security |
Describes electronic standards for own job classification related to the privacy and security of protected information* |
Ensures that individual laboratory programs adhere to electronic security and privacy standards |
Evaluates individual electronic security and privacy standards that the laboratory must meet |
Develops workflows and modules to ensure electronic systems meet security and privacy standards and adhere to regulatory requirements |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 14.00. Customer relationship management: manages customer relationships |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
14.01. Tracking customer and staff feedback |
Describes predefined modules to record customer and staff feedback |
Verifies the implementation of predefined modules to record customer and staff feedback |
Evaluates current and future needs to handle customer relationships and perform ad hoc queries to improve regulatory compliance and business management |
Develops workflow and modules that automate the collection of data to track and to perform ad hoc queries and reporting of customer and staff feedback |
|
INF 14.02. Tracking laboratory errors and information requests |
Describes predefined modules to record laboratory errors and information requests |
Verifies the implementation of predefined modules to record laboratory errors and information requests |
Evaluates current and future needs to track and perform ad hoc queries on laboratory errors and information requests |
Develops workflow and modules that automate the collection of data to track and to perform ad hoc queries and reporting of laboratory errors and information requests |
|
INF 14.03. Tracking corrective actions and reports |
Describes predefined modules that summarize reporting and corrective actions |
Verifies the implementation of predefined modules to summarize corrective actions and generate reports |
Evaluates current and future needs to track, perform ad hoc queries, and provide reports regarding corrective actions |
Develops workflow and modules that automate the collection of data to track and to perform ad hoc queries and reporting of correction actions |
|
INF 15.00. QC and QA management: manages quality control and quality assurance processes |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 15.01. QC data associated with sample results |
Describes predefined modules that collect QC data associated with sample results |
Verifies the electronic set-up, extraction, and transmission of QC data using predefined modules and data capture utilities for automated instruments |
Evaluates automated systems for QC data set up, extraction, and transmission |
Develops automated processes for QC data set up, extraction, and transmission |
|
INF 15.02. Data review and validation |
Lists QC data that are collected electronically to support validation of test results |
Performs review and validation of data |
Validates final data prior to release to customers using configurable rules-based functionality |
Develops workflows and modules to assist in automating the validation of test results |
|
INF 15.03. Data trending |
Enters QC data to support tracking, trending, and analysis of method accuracy and precision |
Verifies the use of automated software to support analysis of QC data related to tracking, trending, and analysis of method accuracy and precision |
Evaluates automated tracking, trending, and analysis of method accuracy and precision |
Develops the workflows and automation processes to support automated tracking, trending, and analysis of method accuracy and precision |
|
INF 15.04. QC reporting |
Uses predefined modules to produce electronic and paper results that include QC data associated with test runs |
Verifies the reporting and evaluation of QC data associated with analytical testing using predefined modules |
Evaluates automated reporting of QC data associated with analytical batches |
Develops reports that meet method requirements and customer needs to capture and deliver QC data in multiple formats and messages |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 15.00. QC and QA management: manages quality control and quality assurance processes |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 15.05. QA and quality management systems (QMS)* |
Describes organizational structure, policies, processes, procedures, and resources that address QA related to informatics activities |
Performs day-to-day oversight of organizational structure, policies, processes, procedures, and resources that address QA- and QMS-related to informatics activities |
Evaluates organizational structure, policies, processes, procedures, and resources that address QA- and QMS-related to informatics activities |
Develops informatics workflows and the organizational structure, policies, processes, procedures, and resources to address QMS and QA related to informatics activities |
|
INF 15.06. Responses to QC data |
Describes data elements needed to support automated auto-alerts, qualifiers, or triggering of responses to QC data |
Verifies that data to support auto-alerts, qualifiers, or triggering of responses to QC data are associated with test results |
Validates data elements using configurable rules-based functionality to provide auto- alerts, qualifiers, or triggering of responses to QC data |
Develops automated workflows to provide auto-alerts, qualifiers, and triggering of responses to QC data |
|
INF 16.00. Laboratory safety and accident investigation: manages laboratory safety and accident investigations |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 16.01. Hazardous materials* management |
Describes electronic data entry and access to hazardous material locations, safety data sheets (SDS),* procedures, disposal records* current practice standards, and master records |
Verifies the central management of hazardous materials using predefined electronic modules for reporting and tracking |
Evaluates the electronic, centralized management of hazardous materials |
Develops an electronic, centralized system to manage hazardous materials |
|
INF 16.02. Incident* tracking |
Describes predefined modules to track incidents |
Ensures staff compliance with electronic reporting of laboratory safety activities and accident investigations |
Manages laboratory electronic safety and accident investigation processes and procedures |
Develops electronic workflows, processes, and procedures to track and manage safety and accident investigations |
|
INF 16.03. Select agent* management |
Describes predefined modules related to the federal Select Agent Program* and registry |
Ensures staff compliance with regulations associated with the federal Select Agent Program and registry |
Evaluates automated processes to adhere to the federal Select Agent Program and registry |
Develops electronic modules that adhere to the federal Select Agent Program and registry |
|
INF 16.04. Hazardous material alerts* |
Describes system alerts associated with hazardous materials |
Verifies the generation of package and sample labels once an alert is received electronically |
Evaluates current and future electronic hazardous material alert requirements |
Develops electronic modules to manage and track activities associated with hazardous material alerts |
|
INF 16.05. Hazardous risk management |
Lists electronic central documents that define laboratory processes related to hazardous risk management |
Verifies that documentation systems track laboratory processes related to hazardous risk management |
Evaluates the documentation systems for laboratory processes related to hazardous risk management |
Develops task workflow analyses to ensure the electronic management of hazardous risks |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 17.00. Laboratory mutual assistance and disaster recovery: manages laboratory mutual assistance and disaster recovery |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 17.01. Continuity of Operations Plan (COOP)* |
Outlines coverage of informatics and IT services within the laboratory's COOP |
Verifies that laboratory program responsibilities are carried out during the implementation of a COOP |
Evaluates the informatics and IT aspects of the laboratory COOP to ensure they are complete and up-to-date |
Develops informatics COOP workflows and procedures to restore informatics and IT support |
|
INF 17.02. COOP contractual instruments |
Describes COOP formal contractual instruments involving informatics |
Verifies the performance of laboratory program informatics responsibilities pertaining to COOP contractual instruments |
Manages the documentation supporting existing informatics contractual instruments pertaining to disaster recovery and mutual assistance through drills |
Develops work plans and project management processes to ensure that comprehensive informatics contractual instruments are in place |
|
INF 17.03. Electronic catalogue of capacities and services |
Explains the importance of a catalogue of electronic capabilities and services, and of schedules for testing their effectiveness during disaster recovery and emergency situations |
Verifies the accuracy and access to a catalogue of electronic capabilities, services, and schedules for testing their effectiveness during disaster recovery and emergency situations |
Evaluates electronic catalogue capabilities, services, and schedules for testing their effectiveness during disaster recovery and emergency situations |
Develops workflows and project management processes to validate the electronic catalogue of capabilities, services, schedules, and testing of effectiveness during disaster recovery and emergency situations |
|
INF 18.00. Core IT products and services: manages core IT hardware, software, and services |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 18.01. Client-side systems and software |
Employs client-side computer systems including LIMS access protocols and routine office software |
Verifies the correct use of LIMS and advanced use of office software |
Evaluates modifications, upgrades, and new releases to laboratory instrument software and systems |
Evaluates client-side options regarding LIMS installation, management, and use |
|
INF 18.02. Electronic communication |
Describes communication tools for electronic information |
Determines content for electronic information communication tools |
Evaluates the use and requirements of electronic information communication tools |
Develops the technology to support electronic information communication tools |
|
INF 18.03. Enterprise-wide LIMS availability |
Describes LIMS management processes |
Verifies LIMS processes for data collection, data processing and reporting for laboratory business needs |
Evaluates LIMS functionality to meet laboratory and customer needs |
Manages the continuous availability and development of an enterprise-wide LIMS to ensure a fully functional and mature system |
|
INF 18.04. Networking |
Describes network access protocols and use of the laboratory network |
Verifies laboratory use of predefined network protocols |
Evaluates the use of networks to support laboratory activities |
Manages the administration of network servers |
|
INF 18.05. IT help desk |
Describes access to available IT support |
Documents the delivery of support to laboratory programs |
Evaluates IT support needed for laboratory operations |
Develops formal contractual instruments, workflows, and project management processes for the delivery of IT support throughout the laboratory |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 18.00. Core IT products and services: manages core IT hardware, software, and services |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 18.06. Software development life cycle (SDLC)* |
Describes the processes to use and improve electronic laboratory workflows and algorithms |
Verifies needs for software process improvements |
Evaluates the resources that impact the SDLC |
Manages the SDLC at the enterprise-level, including change management* |
|
INF 18.07. Enterprise integration engine* |
Lists predefined functions that use an integration engine |
Verifies the use of predefined integration engine modules |
Evaluates integration functions with partners and customers |
Develops automated workflows and processes regarding data capture and use of an integration engine to message data |
|
INF 18.08. Legacy data systems* |
Defines legacy data systems |
Verifies access to, and use of, legacy data systems |
Evaluates the accessibility and searchability of legacy data |
Develops workflows and processes to ensure that legacy data are accessible and searchable |
|
INF 18.09. LIMS communication with third-party data systems |
Describes predefined modules to support LIMS communication with third-party data systems |
Verifies the proper use of predefined modules to support LIMS communication with third-party data systems |
Evaluates the ability of the LIMS to communicate with third-party data systems |
Develops workflows and processes to support LIMS communications with third-party systems |
|
INF 18.10. Access and audit trails |
Describes modules that provide a view of audit trails |
Verifies staff access to electronic audit trails |
Evaluates the ability of the LIMS to manage access and audit trails |
Develops protocols to manage access and audit trails |
|
INF 18.11. Instrument analysis software |
Describes instrument analysis software systems |
Uses instrument analysis software |
Evaluates analytical instrument software |
Develops analytical instrument software systems |
|
INF 18.12. Computer maintenance and troubleshooting |
Identifies basic computer problems |
Performs routine computer maintenance and troubleshooting |
Performs complex computer maintenance and troubleshooting |
Manages the computer maintenance and troubleshooting processes |
|
INF 19.00. Policies and procedures: manages operational, budgeting and funding policies and procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 19.01. Informatics policy |
Describes existing informatics policies |
Ensures laboratory program adherence to existing informatics policies |
Evaluates informatics policies |
Develops processes for informatics policy making |
|
INF 19.02. Change control¶ |
Describes change control processes related to informatics |
Ensures laboratory program adherence to the change control processes related to informatics |
Evaluates the effectiveness of change control processes related to informatics |
Develops informatics policies, processes, and procedures to manage change control and ensure staff compliance |
|
INF 19.03. Documentation for standardized laboratory IT processes |
Provides documentation for standardized laboratory IT processes |
Verifies that paper and electronic documentation of laboratory IT processes are centrally located and accessible |
Evaluates completeness of documentation for standardized laboratory IT processes |
Develops workflows and processes to ensure paper and electronic documentation is centrally located and accessible |
|
INF 19.04. Operational budgeting strategy |
Describes the importance of electronic budgetary processes |
Identifies informatics business needs of the laboratory program for operational budgeting strategizing |
Evaluates the operational budgeting strategy |
Develops a budgeting strategy for the laboratory's informatics systems and services |
|
TABLE 11. (Continued) Public health laboratory competency guidelines: Informatics domain |
||||
|---|---|---|---|---|
|
INF 19.00. Policies and procedures: manages operational, budgeting and funding policies and procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
INF 19.05. Capital budgeting* |
Describes the capital budgeting processes for informatics-related assets* |
Provides the listing of capital budget needs regarding laboratory informatics |
Evaluates capital budgeting needs regarding laboratory informatics |
Develops capital budgets for electronic information systems and services |
|
INF 19.06. Partnership channels |
Lists established stakeholders and partnership channels |
Presents short- and long-term informatics business needs to management staff |
Ensures that laboratory informatics business needs are presented to stakeholders and partnership channels |
Develops partnerships to facilitate funding for informatics |
|
* This term is defined in Appendix B. † All of the tasks, in the proper order, required to carry out a process. § A set of technical rules for the transmission and receipt of information between computers. ¶ A process for implementing changes to software or other information technology solutions using a coordinated approach. |
||||
|
TABLE 12. (Continued) Public health laboratory competency guidelines: Microbiology domain |
|||||
|---|---|---|---|---|---|
|
MCB 2.00. Facilities and safety: works safely with microbiological agents within a laboratory facility* |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 2.03. Personal protective equipment (PPE)* |
Adheres to policies, processes, and procedures regarding PPE use for work related to microbiological agents |
Instructs staff in policies, processes, and procedures regarding PPE use for work related to microbiological agents |
Ensures staff compliance with policies, processes, and procedures regarding PPE use for work related to microbiological agents |
Establishes policies, processes, and procedures regarding PPE use for work related to microbiological agents |
|
|
MCB 2.04. Biosafety cabinets and other engineering controls* |
Adheres to policies, processes, and procedures regarding the use of biosafety cabinets and other engineering controls |
Instructs staff in use of biosafety cabinets and other engineering controls |
Ensures laboratory adherence to biosafety cabinet certification* and staff compliance with policies, processes, and procedures regarding the use of biosafety cabinets and other engineering controls |
Establishes policies, processes, and procedures, including training, to ensure implementation and use of biosafety cabinets and other engineering controls |
|
|
MCB 2.05. Waste management related to microbiological agents |
Adheres to policies, processes, and procedures regarding waste management related to microbiological agents |
Instructs staff in waste management policies, processes, and procedures related to microbiological agents |
Establishes waste management processes and procedures related to microbiological agents |
Oversees the waste management plan* related to microbiological agents |
|
|
MCB 2.06. Decontamination* |
Adheres to policies, processes, and procedures regarding decontamination |
Instructs staff in the policies, processes, and procedures regarding decontamination for different microorganisms |
Ensures staff compliance with policies, processes, and procedures regarding decontamination |
Develops policies, processes, and procedures related to decontamination |
|
|
MCB 2.07. Storage of microbiological materials |
Adheres to policies, processes, and procedures regarding storage of microbiological materials |
Instructs staff in policies, processes, and procedures regarding the storage of microbiological materials for different microorganisms |
Ensures staff compliance with policies, processes, and procedures that address the storage of microbiological materials |
Develops policies, processes, and procedures related to the storage of microbiological materials |
|
|
MCB 3.00. Pre-examination:* assesses microbiological samples* during the pre-examination phase |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 3.01. Sample collection, labeling, and handling |
Describes routine sample collection, labeling, and handling policies, processes, and procedures for microbiological examination |
Consults on nonroutine sample collection, labeling, and handling procedures for microbiological examination |
Monitors staff compliance with established policies, processes, and procedures regarding microbiological sample collection, labeling, and handling |
Oversees the policies, processes, and procedures for sample collection, labeling, and handling for microbiological examination |
|
|
MCB 3.02. Packaging and shipping |
Performs packing and shipping of Category A* and Category B* infectious substances |
Instructs others on packing and shipping of Category A and Category B infectious substances |
Ensures staff compliance with policies, processes, and procedures regarding the packing and shipping of Category A and Category B infectious substances |
Develops policies, processes, and procedures to ensure staff compliance with packing and shipping regulations concerning Category A and Category B infectious substances |
|
|
MCB 3.03. Material transport |
Describes the importance of adhering to established policies, processes, and procedures regarding microbiological material transport |
Instructs others on microbiological material transport policies, processes, and procedures |
Develops microbiological material transport processes and procedures |
Oversees the policies, processes, and procedures regarding microbiological material transport |
|
|
TABLE 12. (Continued) Public health laboratory competency guidelines: Microbiology domain |
|||||
|---|---|---|---|---|---|
|
MCB 3.00. Pre-examination:* assesses microbiological samples* during the pre-examination phase |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 3.04. Biological threats |
Describes policies, processes, and procedures for the identification, handling, safety, appropriateness and triage of samples containing agents of concern |
Adheres to policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing agents of concern |
Ensures staff compliance with policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing agents of concern |
Oversees the policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing agents of concern |
|
|
MCB 3.05. Accessioning and receipt |
Performs procedures for accessioning and receipt of microbiological samples |
Instructs staff in policies, processes, and procedures regarding accessioning and receipt of microbiological samples |
Develops processes and procedures for microbiological sample accessioning and receipt |
Oversees the policies, processes, and procedures regarding microbiological sample accessioning and receipt |
|
|
MCB 3.06. Sample tracking |
Performs systematic tracking of microbiological samples from receipt to final disposition |
Instructs staff on policies, processes, and procedures for the systematic tracking of microbiological samples from receipt to final disposition |
Develops processes and procedures for the systematic tracking of microbiological samples from receipt to final disposition |
Oversees the policies, processes, and procedures for the systematic tracking of microbiological samples from receipt to final disposition |
|
|
MCB 3.07. Sample evaluation and appropriateness* for testing |
Assesses sample appropriateness for a particular microbiological examination |
Instructs others in the appropriateness of routine and nonroutine samples for microbiological examination |
Develops processes and procedures for assessment of routine and nonroutine sample appropriateness for microbiological examination |
Oversees the policies, processes, and procedures regarding assessment of routine and nonroutine sample appropriateness for microbiological examination |
|
|
MCB 3.08. Testing workflow§ |
Adheres to policies, processes, and procedures regarding testing workflow |
Instructs staff in policies, processes, and procedures regarding testing workflow |
Ensures staff compliance with policies, processes, and procedures related to testing workflow |
Establishes policies, processes, and procedures related to testing workflow |
|
|
MCB 3.09. Nucleic Acid Amplification Tests (NAAT) workflow (facility specific) |
Adheres to policies, processes, and procedures regarding NAAT workflow |
Instructs staff in policies, processes, and procedures regarding NAAT workflow, including pre- and postamplification areas |
Ensures staff compliance with policies, processes, and procedures regarding NAAT workflow |
Establishes policies, processes, and procedures for NAAT workflow |
|
|
MCB 3.10. Sample set-up |
Performs sample set-up procedures for microbiological examinations |
Instructs staff in sample set-up for microbiological examinations |
Develops processes and procedures regarding sample set-up for microbiological examinations |
Oversees the policies, processes, and procedures regarding sample set-up for microbiological examinations |
|
|
MCB 3.11. Sample storage and handling |
Performs procedures for microbiological sample storage and handling prior to examination |
Instructs staff in procedures for microbiological sample storage and handling prior to examination |
Develops processes and procedures for microbiological sample storage and handling prior to examination |
Oversees the policies, processes, and procedures regarding microbiological sample storage and handling prior to examination |
|
|
MCB 3.12. Epidemiologic collaboration |
Describes which microbiological examination requests require epidemiologic notification and consultation |
Reports to epidemiologists when microbiological examination requests warrant notification |
Ensures staff compliance with policies, processes, and procedures for notification and consultation with epidemiologists regarding microbiological examination requests |
Oversees the policies, processes, and procedures for notification and consultation with epidemiologists regarding microbiological examination requests |
|
|
TABLE 12. (Continued) Public health laboratory competency guidelines: Microbiology domain |
|||||
|---|---|---|---|---|---|
|
MCB 4.00. Examination:* assesses microbiological samples during the examination phase |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 4.01. Preparation of culture media |
Prepares culture media according to policies, processes, and procedures |
Instructs staff on the preparation of culture media |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for media preparation |
Oversees the policies, processes, and procedures for media preparation |
|
|
MCB 4.02. Selection of media |
Selects media according to procedures |
Instructs staff in the media selection process |
Ensures staff compliance with policies, processes and procedures regarding quality practices for media selection |
Oversees the policies, processes, and procedures regarding media selection |
|
|
MCB 4.03. Culture inoculation |
Performs culture inoculation using aseptic techniques |
Instructs staff in how to inoculate cultures using aseptic technique |
Ensures staff compliance with policies, processes and procedures regarding quality practices for culture inoculation |
Oversees the policies, processes, and procedures regarding culture inoculation |
|
|
MCB 4.04. Microscopic examination with morphological characteristics |
Recognizes the morphological characteristics of different organisms |
Instructs staff in morphological identification and differentiating organisms from artifacts |
Ensures staff compliance with policies, processes and procedures regarding quality practices for morphological identification |
Oversees the policies, processes, and procedures regarding morphological identification |
|
|
MCB 4.05. Culture growth characteristics |
Recognizes growth characteristics of microorganisms |
Instructs staff in identifying growth characteristics of microorganisms |
Ensures staff compliance with policies, processes and procedures regarding quality practices for identifying microorganism growth characteristics |
Oversees the policies, processes, and procedures for interpretation of microorganism growth characteristics |
|
|
MCB 4.06. Manual identification* and susceptibility testing* methods |
Performs identification and susceptibility testing using manual methods |
Instructs staff in the performance of identification and susceptibility testing using manual methods |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for performing identification and susceptibility testing using manual methods |
Oversees the policies, processes, and procedures regarding the performance of identification and susceptibility testing using manual methods |
|
|
MCB 4.07. Automated identification* and susceptibility testing* systems |
Performs identification and susceptibility testing using automated systems |
Instructs staff in the performance of identification and susceptibility testing using automated systems, including how to utilize algorithms to determine additional testing |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for performing identification and susceptibility testing using automated systems |
Oversees the policies, processes, and procedures regarding the performance of identification and susceptibility testing using automated systems |
|
|
MCB 4.08. Agent-specific antigen detection* |
Performs antigen detection methods |
Instructs staff in how to perform antigen detection methods |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for antigen detection methods |
Oversees the policies, processes, and procedures for antigen detection |
|
|
MCB 4.09. Nucleic acid (NA) sequencing of infectious agents |
Performs NA sequencing of infectious agents |
Instructs staff in the performance of NA sequencing for the identification of infectious agents |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for NA sequencing of infectious agents, including the selection and utilization of databases |
Oversees the policies, processes, and procedures regarding NA sequencing and sequence-based identification |
|
|
TABLE 12. (Continued) Public health laboratory competency guidelines: Microbiology domain |
|||||
|---|---|---|---|---|---|
|
MCB 4.00. Examination:* assesses microbiological samples during the examination phase |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 4.10. Strain typing methods* |
Performs strain typing |
Instructs staff in how to perform strain typing |
Ensures staff compliance with policies, processes, and procedures regarding quality practices for strain typing |
Oversees the policies, processes, and procedures regarding strain typing |
|
|
MCB 4.11. Rule-out testing for agents of concern |
Explains the policies, processes, and procedures regarding rule-out testing and referral |
Performs rule-out testing and referral for identification, confirmation, and characterization of agents of concern |
Ensures the laboratory responds quickly to needs for rapid testing with timely notification and secure messaging of results |
Oversees the policies, processes, and procedures regarding rule-out testing and referral |
|
|
MCB 4.12. Quality control (QC)* analysis |
Performs QC activities |
Interprets QC data prior to reporting results |
Examines QC data over time to establish QC ranges and limits |
Ensures the QC program adheres to regulatory requirements* |
|
|
MCB 5.00. Postexamination:* performs postexamination procedures of microbiological testing |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 5.01. QC evaluation |
Assembles QC data for evaluation |
Evaluates QC data for a given data reporting period |
Ensures staff compliance with established policies, processes, and procedures for QC evaluation activities |
Oversees the policies, processes, and procedures related to QC evaluation activities |
|
|
MCB 5.02. Test analysis and results interpretation |
Assembles test data for review and action |
Analyzes test data |
Interprets complex or ambiguous results |
Oversees the policies, processes, procedures, and algorithms related to data analysis and results interpretation |
|
|
MCB 5.03. Results reporting and data release |
Adheres to policies, processes and procedures related to reporting and release of examination results and notifiable results* |
Instruct staff in the policies, processes, and procedures related to reporting and release of examination results and notifiable results |
Ensures staff compliance with policies, processes and procedures related to reporting and release of examination results and notifiable results |
Oversees the policies, processes, and procedures, related to reporting and release of examination results and notifiable results to partners |
|
|
MCB 5.04. Quality assurance (QA)* |
Explains the differences between QA and QC |
Collects data for reporting on QA indicators |
Evaluates QA indicator data |
Oversees the policies, processes, and procedures related to QA |
|
|
TABLE 12. (Continued) Public health laboratory competency guidelines: Microbiology domain |
|||||
|---|---|---|---|---|---|
|
MCB 6.00. Regulatory compliance: ensures regulatory compliance |
|||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
|
MCB 6.01. Nonconforming event (NCE)* tracking |
Recognizes NCEs in laboratory processes |
Documents NCEs on discovery for implementation of corrective actions |
Analyzes NCEs for corrective actions and documentation |
Designs a management system for NCEs |
|
|
MCB 6.02. Proficiency testing (PT)* and alternative assessment* |
Performs PT and alternative assessment |
Reviews PT and alternative assessment results |
Monitors to ensure the PT and alternative assessment program meets regulatory requirements |
Oversees the policies, processes, and procedures related to PT and alternative assessments |
|
|
MCB 6.03. Method validation* and performance verification* |
Participates in performance of method validation and performance verification |
Compiles results of method validation and performance verification |
Evaluates method validation and performance verification results |
Oversees the policies, processes, and procedures related to method validation and performance verification |
|
|
MCB 6.04. Development and validation of laboratory-developed tests (LDTs)* |
Participates in the development of LDTs |
Evaluates LDT validation data |
Creates processes and procedures for the development and validation of LDTs |
Oversees the policies, processes, and procedures regarding the development and validation of LDTs |
|
|
MCB 6.05. Select agents* |
Describes the policies, processes, and procedures related to the federal Select Agent Program,* including the securing, safe handling, and testing of select agents and the documentation of activities |
Complies with policies, processes, and procedures related to the federal Select Agent Program, including the securing, safe handling, and testing of select agents and the documentation of activities |
Implements policies, processes, and procedures regarding select agent security, biosafety, testing, and incident response plans* |
Oversees select agent security, biosafety, testing, and incident response plans to ensure alignment with select agent regulations |
|
|
* This term is defined in Appendix B. † The evaluation of the probability and consequences of exposure to a given hazard, with the intent to reduce the risk by establishing the appropriate hazard controls to be used. § Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures.
|
|||||
|
TABLE 13. (Continued) Public health laboratory competency guidelines: Chemistry domain |
||||
|---|---|---|---|---|
|
CHM 2.00. Facilities and safety: works safely with hazardous materials* within a laboratory facility* |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 2.01. Chemical hazards |
Recognizes chemical hazards and chemical hazard communication* |
Instructs others on chemical hazards and hazard communication |
Implements hazard communication procedures and training |
Develops strategies to reduce chemical hazards based on risk assessments† |
|
CHM 2.02. Safe work practices* |
Adheres to safe work practices related to chemical hazards |
Instructs others in safe work practices, policies, and procedures related to chemical hazards |
Ensures staff compliance with policies,* processes, and procedures related to safe work practices related to chemical hazards |
Creates a culture of safety by ensuring that policies, processes, and procedures regarding safe work practices related to chemical hazards are aligned with current standards and regulatory requirements |
|
CHM 2.03. Personal protective equipment (PPE)* |
Adheres to policies, processes, and procedures regarding PPE use |
Instructs staff in the use of PPE |
Ensures that staff are trained and comply with policies, processes, and procedures regarding the use of PPE |
Establishes policies, processes, and procedures regarding the use of PPE |
|
CHM 2.04. Engineering controls* |
Adheres to policies, processes, and procedures regarding the use of engineering controls |
Instructs staff in the use of engineering controls |
Ensures that staff are trained and comply with policies, processes, and procedures regarding the use of engineering controls |
Establishes policies, processes, and procedures regarding the use of engineering controls |
|
CHM 2.05. Waste management related to samples* |
Adheres to policies, processes, and procedures related to sample waste management |
Instructs staff in sample waste management policies, processes, and procedures |
Establishes sample waste management policies, processes, and procedures |
Oversees the sample waste management plan* |
|
CHM 3.00. Pre-examination:* performs chemistry pre-examination procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 3.01. Sample collection, labeling, and handling |
Describes routine sample collection, labeling, and handling policies, processes, and procedures for chemical examination |
Consults on nonroutine sample collection, labeling, and handling procedures for chemical examination |
Monitors staff compliance with established policies, processes, and procedures regarding chemical sample collection, labeling, and handling |
Oversees the policies, processes, and procedures for the collection and handling of samples for chemical examination |
|
CHM 3.02. Packaging and shipping |
Performs the packing and shipping of hazardous samples |
Instructs others on packing and shipping of hazardous samples |
Ensures staff compliance with policies, processes, and procedures regarding the packing and shipping of hazardous samples |
Develops policies, processes, and procedures to ensure staff compliance with packing and shipping regulations concerning hazardous samples |
|
CHM 3.03. Material transport |
Describes the importance of adhering to established policies, processes, and procedures regarding transport of materials for chemical examination |
Instructs others on material transport policies, processes, and procedures |
Develops material transport processes and procedures |
Oversees the policies, processes, and procedures for the transport of materials for chemical examination |
|
CHM 3.04. Chemical threats |
Describes the policies, processes, and procedures for the identification, handling, safety, appropriateness and triage of samples containing chemical agents of concern |
Adheres to policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing chemical agents of concern |
Ensures staff compliance with policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing chemical agents of concern |
Oversees the policies, processes, and procedures regarding the identification, handling, safety, appropriateness and triage of samples containing chemical agents of concern |
|
TABLE 13. (Continued) Public health laboratory competency guidelines: Chemistry domain |
||||
|---|---|---|---|---|
|
CHM 3.00. Pre-examination:* performs chemistry pre-examination procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 3.05. Accessioning and receipt |
Performs procedures for sample accessioning and receipt |
Instructs staff on policies, processes, and procedures regarding sample accessioning and receipt |
Manages the policies, processes, and procedures for sample accessioning and receipt |
Designs policies, processes, and procedures regarding sample accessioning and receipt |
|
CHM 3.06. Sample tracking |
Performs systematic tracking of samples from receipt to final disposition |
Instructs staff on policies, processes, and procedures for the systematic tracking of samples from receipt to final disposition |
Manages the policies, processes, and procedures for the systematic tracking of samples from receipt to final disposition |
Designs polices, processes, and procedures for the systematic tracking of samples from receipt to final disposition |
|
CHM 3.07. Sample evaluation and appropriateness* for testing |
Assesses appropriateness of routine samples for chemical examination |
Instructs others in appropriateness of routine and nonroutine samples for chemical examination |
Manages the policies, processes, and procedures for assessment of routine and nonroutine sample appropriateness for chemical examination |
Establishes policies, processes, and procedures regarding the assessment of routine and nonroutine sample appropriateness for chemical examination |
|
CHM 3.08. Testing workflow§ |
Adheres to policies, processes, and procedures regarding testing workflow |
Instructs staff in policies, processes, and procedures regarding testing workflow |
Ensures laboratory processes and procedures include use of testing workflow |
Develops policies, processes, and procedures related to testing workflow |
|
CHM 3.09. Sample processing |
Performs sample processing procedures for routine chemical examinations |
Performs sample processing for complex chemical examinations |
Instructs staff on sample processing for routine and complex chemical examinations |
Develops policies, processes, and procedures regarding sample processing for routine and complex chemical examinations |
|
CHM 3.10. Pre-examination sample storage and handling |
Performs procedures for sample storage and handling prior to examination |
Instructs staff in procedures for sample storage and handling prior to examination |
Manages processes and procedures for sample storage and handling prior to examination |
Designs policies, processes, and procedures regarding sample storage and handling prior to examination |
|
CHM 3.11. Epidemiologic collaboration |
Describes which chemical examination requests require epidemiologic notification and consultation |
Reports to epidemiologists when chemical examination requests warrant notification |
Manages the policies, processes, and procedures for notification and consultation with epidemiologists regarding chemical examination requests |
Designs policies, processes, and procedures regarding notification and consultation with epidemiologists regarding chemical examination requests |
|
CHM 4.00. Examination:* performs chemistry examination procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 4.01. Examination |
Performs chemistry examination procedures |
Instructs staff in chemistry examination procedures |
Monitors staff compliance with chemistry examination policies, processes, and procedures |
Oversees chemistry examination policies, processes, and procedures |
|
CHM 4.02. Chemical extractions |
Performs routine chemical extraction methods |
Performs nonroutine chemical extractions |
Selects chemical extractions for an identified purpose |
Develops new and improved types of chemical extractions |
|
CHM 4.03. Quality control (QC)* analysis |
Performs QC activities |
Interprets QC data prior to reporting results |
Examines QC data over time to establish QC ranges and limits |
Ensures the QC program adheres to regulatory requirements* |
|
CHM 4.04. Equipment troubleshooting |
Identifies basic laboratory equipment* problems |
Corrects equipment problems or failures |
Monitors equipment functioning during its lifecycle |
Develops equipment troubleshooting processes and procedures |
|
TABLE 13. (Continued) Public health laboratory competency guidelines: Chemistry domain |
||||
|---|---|---|---|---|
|
CHM 4.00. Examination:* performs chemistry examination procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 4.05. Sample storage and handling after examination |
Performs procedures for sample storage and handling after examination |
Instructs staff in procedures for sample storage and handling after examination |
Manages processes and procedures for sample storage and handling after examination |
Designs policies, processes, and procedures for sample storage and handling after examination |
|
CHM 5.00. Postexamination:* performs chemistry postexamination procedures |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 5.01.QC evaluation |
Assembles QC data for evaluation |
Evaluates QC data for a given data reporting period |
Ensures staff compliance with established policies, processes, and procedures for QC evaluation activities |
Oversees the policies, processes, and procedures related to QC evaluation activities |
|
CHM 5.02. Test analysis and results interpretation |
Assembles test data for review and action |
Analyzes test data |
Interprets complex or ambiguous results |
Oversees the policies, processes, procedures, and algorithms related to data analysis and results interpretation |
|
CHM 5.03. Results reporting and data release |
Adheres to policies, processes and procedures related to reporting and release of examination results and notifiable results* |
Instruct staff in the policies, processes, and procedures related to reporting and release of examination results and notifiable results |
Ensures staff compliance with policies, processes and procedures related to reporting and release of examination results and notifiable results |
Oversees the policies, processes, and procedures, related to reporting and release of examination results and notifiable results to partners |
|
CHM 5.04. Quality assurance (QA)* |
Explains the differences between QA and QC |
Collects data for reporting on QA indicators |
Evaluates QA indicator data |
Oversees the policies, processes, and procedures related to QA |
|
CHM 6.00. Regulatory compliance: ensures regulatory compliance |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
CHM 6.01. Nonconforming event (NCE)* tracking |
Recognizes NCEs in laboratory processes |
Documents NCEs on discovery for implementation of corrective actions |
Analyzes NCEs for corrective actions and documentation |
Designs a management system for NCEs |
|
CHM 6.02. Proficiency testing (PT)* and alternative assessment* |
Performs PT and alternative assessment |
Reviews PT and alternative assessment results |
Monitors to ensure the PT and alternative assessment program meets regulatory requirements |
Oversees the policies, processes, and procedures related to PT and alternative assessments |
|
CHM 6.03. Method validation* and performance verification* |
Participates in performance of method validation and performance verification |
Compiles results of method validation and performance verification |
Evaluates method validation and performance verification results |
Oversees the policies, processes, and procedures related to method validation and performance verification |
|
CHM 6.04. Development and validation of laboratory-developed tests (LDTs)* |
Participates in the development of LDTs |
Evaluates LDT validation data |
Creates processes and procedures for the development and validation of LDTs |
Oversees the policies, processes, and procedures regarding the development and validation of LDTs |
|
* This term is defined in Appendix B. † The evaluation of the probability and consequences of exposure to a given hazard, with the intent to reduce the risk by establishing the appropriate hazard controls to be used. § Sequential steps in a laboratory's activities that transform a submitter's test order into the laboratory information captured in the report of results, including pre-examination, examination, and postexamination procedures. |
||||
|
TABLE 14. (Continued) Public health laboratory competency guidelines: Bioinformatics domain |
||||
|---|---|---|---|---|
|
BIO 3.00. Data analysis: analyzes biological data |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
BIO 3.01. Data analysis |
Selects which existing tools and algorithms to use for any given analysis |
Determines options and parameters of tools to meet specified needs of a given data analysis |
Implements new software tools to address unmet needs or improve current processes* |
Creates stand-alone analysis tools |
|
BIO 3.02. Data interpretation |
Identifies data pertinent to the analysis problem |
Formulates results of analyses, including information in the form of graphs, charts, and tables |
Interprets results within the context of the analysis problem |
Generates hypotheses to predict future implications based on the evaluation of data analyses |
|
BIO 3.03. Data visualization and representation |
Uses data visualization and representation tools to present results of data analyses |
Selects visualization and representation tools for specified bioinformatics problems |
Evaluates representation and visualization tools for summarizing data analyses |
Modifies existing visualization and representation tools to provide insight into bioinformatics analyses |
|
BIO 3.04. Communication |
Discusses bioinformatics with other scientists within their institution |
Initiates bioinformatics collaborations with colleagues |
Facilitates knowledge-sharing with stakeholders |
Contributes to new findings and meaningful advancements in bioinformatics through the evaluation and sharing of knowledge |
|
BIO 4.00. Data management: conducts data management, storage, and retrieval |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
BIO 4.01. Data structures* |
Demonstrates basic knowledge of data structures |
Applies knowledge of data structures to relevant problems |
Manipulates data structures to address biological problems |
Develops new data structures |
|
BIO 4.02. Data management |
Describes data management techniques |
Applies knowledge of data management techniques to relevant problems |
Evaluates data management techniques |
Develops new data management techniques |
|
BIO 4.03. Data storage and retrieval |
Describes data storage and retrieval techniques |
Applies knowledge of data storage and retrieval techniques |
Evaluates data storage and retrieval techniques |
Develops new data storage and retrieval techniques |
|
BIO 4.04. Allocation of computing resources |
Describe available computing resources and capacity |
Allocates computing resources |
Manages allocation of multiple computing resources |
Develops new methods for allocation of computing resources |
|
* This term is defined in Appendix B. |
||||
|
TABLE 15. (Continued) Public health laboratory competency guidelines: Research domain |
||||
|---|---|---|---|---|
|
RES 2.00. Ethical conduct: ensures the ethical and responsible conduct of research |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 2.01. Ethical conduct in research* |
Complies with policies,* processes, and procedures* related to ethical conduct in research |
Instructs others in policies, processes, and procedures regarding ethical conduct in research |
Ensures staff compliance to policies and procedures related to ethical research practices |
Oversees the policies, processes, and procedures for implementing and maintaining ethical research practices |
|
RES 2.02. Human and nonhuman subjects |
Complies with policies, processes, and procedures related to doing research in human and nonhuman subjects |
Instructs others in policies, processes, and procedures related to doing research in human and nonhuman subjects |
Ensures staff compliance to policies and procedures related to doing research in human and nonhuman subjects |
Oversees the policies, processes, and procedures for implementing and maintaining ethical practices* related to doing research in human and nonhuman subjects |
|
RES 2.03. Collaboration |
Complies with established agreements with collaborators |
Describes complexities regarding issues of collaboration, including authorship |
Ensures staff compliance with established agreements for research collaboration |
Builds research collaborations |
|
RES 2.04. Sharing research data |
Complies with established agreements pertaining to research data sharing and the use of intellectual property |
Describes issues that might arise pertaining to data ownership and the sharing of data |
Ensures staff compliance with established agreements regarding data sharing |
Establishes guidelines for sharing research data |
|
RES 3.00. Research foundation: integrates scientific and technical knowledge for use as a foundation for research |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 3.01. Literature searches |
Performs basic literature searches using a limited number of sources |
Performs complex searches, aggregating results from multiple sources |
Validates appropriateness of how searches are performed |
Rectifies gaps in data generated from searches |
|
RES 3.02. Critique of scientific literature |
Reads scientific and technical literature relevant to own work |
Assesses quality of literature and pertinence to own work |
Synthesizes scientific evidence derived from literature |
Evaluates scientific literature and data to determine impact on laboratory research programs |
|
RES 3.03. Statistical concepts and tests |
Recognizes meaning of common statistical concepts and tests |
Applies appropriate statistical concepts and tests in performance of research |
Interprets statistical tests and concepts used in literature |
Critiques statistical tests and concepts used in literature |
|
RES 3.04. Study designs |
Explains characteristics of common study designs |
Recognizes strengths and limitations of study designs |
Considers study design characteristics when planning research activities |
Evaluates evidence-based research guidelines to select or develop study designs |
|
RES 3.05. Scientific and technical concepts and procedures |
States scientific and technical concepts and procedures |
Discusses scientific and technical concepts and procedures |
Critiques scientific and technical concepts and procedures |
Generates novel scientific and technical concepts and procedures |
|
RES 3.06. Emerging trends |
States latest scientific and technical advances relevant to current research |
Discusses latest scientific and technical advances relevant to current research |
Identifies emerging trends in scientific and technical advances and possible impact to laboratory |
Analyzes emerging trends in scientific and technical advances to make decisions regarding impact on laboratory |
|
TABLE 15. (Continued) Public health laboratory competency guidelines: Research domain |
||||
|---|---|---|---|---|
|
RES 4.00. Testing methodology development: develops new testing methodology |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 4.01. New testing methodologies |
States the purpose of each step in existing testing methodologies employed |
Describes limitations of existing methodologies |
Proposes concepts for improved methodologies |
Oversees plans for the development of new methodologies |
|
RES 4.02. Pilot testing, method validation,* and performance verification* |
Contributes to pilot testing, method validation, or performance verification |
Performs pilot testing, method validation, or performance verification |
Designs strategies for pilot testing, method validation, or performance verification |
Oversees pilot testing, method validation, and performance verification studies |
|
RES 4.03. New methodology application |
Participates in implementation of new methodologies |
Implements new methodologies into laboratory practice |
Manages implementation of new methodologies |
Oversees the implementation of new methodologies within the laboratory |
|
RES 5.00. Research project execution: conducts research to address a public health issue or answer a public health question |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 5.01. Research project design |
Explains how own tasks and activities support specific research projects |
Summarizes the public health issues and research questions addressed by specific research projects |
Designs research projects |
Oversees research projects to address the identified public health issues or questions |
|
RES 5.02. Experimental strategy and design |
States the purpose of each step performed in individual experiments |
Explains the overall experimental strategy |
Designs individual experiments |
Generates the overall experimental strategy and hypotheses for specific research projects |
|
RES 5.03. Conduct of experiments |
Uses established research protocols† |
Provides input regarding research protocols and the conduct of experiments |
Develops research protocols to guide the conduct of experiments |
Oversees the conduct of experiments |
|
RES 6.00. Research data management, analysis, and application: conducts research according to professional standards of data management, analysis, and application |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 6.01. Data collection and quality* |
Records experimental procedures and data |
Monitors quality and integrity of recorded information and data |
Designs data collection and entry methods that meet data quality standards |
Develops policies, processes, and procedures to ensure data quality and integrity |
|
RES 6.02. Data management |
Complies with policies and procedures for data management |
Assists with the management of data for individual experiments |
Manages project data |
Oversees the management of research data for the laboratory |
|
RES 6.03. Data analysis and results interpretation |
Assists with data analysis of individual experiments |
Analyzes project data |
Interprets data for individual research projects |
Oversees data analysis plans and results interpretation for the laboratory's research projects |
|
RES 6.04. Data summaries |
Describes data tables and graphs |
Summarizes experimental data using multiple formats |
Develops outlines and formats for data summaries |
Critiques data summaries |
|
RES 6.05. Application of research findings to current research |
States laboratory's research findings |
Examines the laboratory's research data to determine its significance in the context of the scientific literature |
Implements integration of internal and external research findings into laboratory's research practices |
Oversees integration of internal and external research findings into revised research agenda, objectives, and/or experimental strategies |
|
TABLE 15. (Continued) Public health laboratory competency guidelines: Research domain |
||||
|---|---|---|---|---|
|
RES 7.00. Dissemination of research findings: disseminates research findings |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 7.01. Meeting and conference presentations |
Attends institutional scientific presentations |
Presents research to colleagues within the organization or via poster at external events |
Presents research via oral presentation for discipline-wide audiences |
Serves as an invited speaker at external meetings and conferences |
|
RES 7.02. Manuscript preparation |
Assists in manuscript drafting and editing |
Drafts sections of research manuscripts |
Publishes as first author or senior author in journals of the discipline |
Publishes as senior author for multi-laboratory or multi-institutional research projects or in high-impact interdisciplinary journals |
|
RES 7.03. Manuscript peer review process |
Reads drafts of manuscripts submitted for peer review |
Informally critiques manuscripts submitted for peer review |
Participates in the peer review process as a formal reviewer |
Participates in the manuscript peer review process as member of the editorial board |
|
RES 8.00. Translation: translates research findings to public health practice |
||||
|
Subcompetency |
Beginner |
Competent |
Proficient |
Expert |
|
RES 8.01. Translation of research findings into public health practice |
States research findings as they relate to current public health practices |
Describes implications of research findings on public health practices |
Directs translation of research findings to public health practices |
Oversees translation of research discoveries into meaningful changes in public health practices |
|
* This term is defined in Appendix B. † A detailed plan for conducting a scientific procedure. |
||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


