Division of Bacterial Diseases (DBD) News Bulletin
This website is archived for historical purposes and is no longer being maintained or updated.
January 4, 2012: Content on this page kept for historical reasons.
In This Issue
A Year in Review
- Quarter 1
- Quarter 2
- Quarter 3
- Quarter 4
- 2012 Events
Winter 2011
Quarter 1
In January, new meningococcal conjugate vaccine (MCV4) recommendations were published in MMWR. Now recommended: 1) a booster dose at age 16 years and 2) a 2-dose primary series for 2 through 54 year-olds with certain medical conditions and for adolescents with HIV infection.
The Streptococcus Laboratory applied classical bacteriology and molecular diagnostics/typing in tracing a meningitis outbreak strain to the oral flora of a radiology physician assistant for an investigation started in late 2010. The Strep Lab has been using multilocus sequencing technology for another ongoing investigation in 2011 involving injections from contaminated pre-prepared avastin syringes that caused severe eye infections in numerous people from two states. The Strep Lab has already discovered that 4 strains from 3 different species were identified from both sources (contaminated syringes and vitreous humor of patients), indicating the probable causal link.

Photo: MVPDB’s Nancy Messonnier accepting the 2011 Philip R. Horne Award from NCIRD Director Anne Schuchat and Surgeon General Regina Benjamin at the National Immunization Conference in Washington, DC.
In January, DBD learned of an increased risk for febrile seizures in children concomitantly receiving influenza and 13-valent pneumococcal conjugate vaccines (PCV13). DBD worked with the Immunization Safety Office, Influenza Division, and Advisory Committee on Immunization Practices (ACIP) work groups to review the data and develop recommendations. Following review by ACIP, materials were developed for parents and providers.
In February, DBD released 2 pertussis training videos illustrating the appropriate collection and handling techniques for nasopharyngeal swab and aspirate procedures. In light of the 2010 pertussis increase, clinicians are increasingly ordering tests for pertussis. Appropriate specimen collection is vital to obtaining accurate diagnostic results.
In February, ACIP made revised recommendations on use of Tdap vaccine and use of postexposure antimicrobial prophylaxis for healthcare personnel. Revised recommendations on use of Tdap in healthcare personnel incorporate the changes made by ACIP at the October 2010 meeting and support direct language to remove barriers to facilitate the uptake of Tdap.
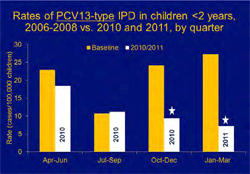
Image: Graph showing early impact of PCV13, as presented by RDB’s Matt Moore at IDSA in October. Evidence suggests PCV13 is already reducing the number of serious infections in children.
March marked a year of PCV13 introduction in the U.S. DBD continues to assess data from 12 geographic regions participating in its ongoing PCV13 Vaccine Effectiveness Evaluation. Early evidence suggests PCV13 is already reducing the number of serious infections in children. See graph at left.
In March, at the 45th National Immunization Conference held in Washington, DC, 10 presentations were made by DBD staff. The 2011 Philip R. Horne Award was presented to Nancy Messonnier for unparalleled scientific contribution and leadership in advancing the mission of NCIRD and the prevention and control of vaccine-preventable diseases, and for exceptional mentorship and staff development. See photo at top.
American Recovery and Reinvestment Act (Recovery Act) funded studies on vaccine effectiveness of pneumococcal and meningococcal vaccines continued throughout 2011. These studies will carry on in 2012 with funding from the Patient Protection and Affordable Care Act and the Vaccines for Children Program.
Director’s Spotlight
Regards from Rana
Dear colleagues,
In this current Bulletin, we attempt to summarize some of the major activities our division participated in or conducted during 2011. This Bulletin highlights the breadth of our work and its important impact on public health both in the U.S. and around the world!
Your hard work and tireless commitment continues to save lives and advance the knowledge about our diseases every day. I would like to thank you for all you did this year and wish you, your families and loved ones healthy and happy Holidays. All the best for 2012!
Rana
- Page last reviewed: January 4, 2012 (archived document)
- Content source:


 ShareCompartir
ShareCompartir