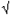In the Absence of SARS-CoV Transmission Worldwide: Guidance for Surveillance, Clinical and Laboratory Evaluation, and Reporting
Version 2
Printer friendly version [15 pages]
This is an updated version of a document first issued by CDC in December 2003. The document provides guidance for surveillance, clinical and laboratory evaluation, and reporting in the setting of no known person-to-person transmission of SARS-CoV worldwide. Recommendations are derived from Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
I. Background
Summary of Changes in Version 2
This version of the guidance document clarifies that the recommendations apply to situations in which no known person-to-person transmission of SARS-CoV is occurring in the world. Some wording has also been revised for consistency with the companion documents, Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS), and Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness.
Severe acute respiratory syndrome (SARS) came to global attention in February 2003, when officials in China informed the World Health Organization (WHO) about 305 cases of atypical pneumonia that had occurred in Guangdong Province. By the time the new infectious disease was declared contained in July 2003, more than 8,000 cases and 780 deaths had been reported from 29 countries worldwide. Since then, active global surveillance for SARS-associated coronavirus (SARS-CoV) disease in humans has detected no laboratory-confirmed person-to-person transmission of SARS-CoV.
No one knows if, when, or where person-to-person transmission of SARS-CoV will recur. However, the rapidity of spread of infection and the high levels of morbidity and mortality associated with SARS-CoV call for careful monitoring for the recurrence of transmission and preparations for the rapid implementation of control measures. The 2003 global outbreaks demonstrated the ease with which SARS-CoV can seed and spread in human populations when cases remain undetected or when infected persons are not cared for in controlled environments that reduce the risk of transmission to others. The two laboratory-acquired infections and the recent cases in Southern China show that SARS-CoV continues to be a threat. Early detection of SARS cases and contacts, plus swift and decisive implementation of containment measures, are therefore essential to prevent transmission. Although the United States had only a limited SARS-CoV outbreak during the 2003 epidemic -- with only eight laboratory-confirmed cases and no significant local spread -- the U.S. population is clearly vulnerable to the more widespread, disruptive outbreaks experienced in other countries. During this period of no known person-to-person transmission of SARS-CoV in the world, healthcare and public health officials must therefore do what they can to prepare for the possibility that SARS-CoV transmission may recur.
This document provides guidance for surveillance, clinical and laboratory evaluation, and reporting in the setting of no known person-to-person transmission of SARS-CoV worldwide. Recommendations are derived from Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS). If such transmission recurs anywhere in the world, CDC will promptly review all available information and provide additional guidance via the Health Alert Network (HAN), Epi-X, and partner organizations. Current information will also be posted on CDC's SARS website.
Top of PageII. Clinical Features of SARS-CoV Disease
The median incubation period for SARS-CoV appears to be approximately 4 to 6 days; most patients become ill within 2 to 10 days after exposure. Early clinical features of SARS-CoV disease can be similar to other viral illnesses and are not sufficiently distinct to enable diagnosis by signs and symptoms alone. The illness usually begins with systemic symptoms such as fever, headache, and myalgias. Respiratory complaints often develop 2 to 7 days after illness onset and usually include a non-productive cough and dyspnea. Upper respiratory symptoms such as rhinorrhea and sore throat may occur but are uncommon. Almost all patients with laboratory evidence of SARS-CoV disease evaluated to date developed radiographic evidence of pneumonia by day 7-10 of illness, and most (70% -90%) developed lymphopenia. The overall case-fatality rate of approximately 10% can increase to >50% in persons older than age 60.
Key Clinical Features of SARS-CoV Disease
- Incubation period of 2-10 days
- Early systemic symptoms followed within 2-7 days by dry cough and/or shortness of breath, often without upper respiratory tract symptoms
- Development of radiographically confirmed pneumonia by day 7-10 of illness
- Lymphopenia in most cases
III. Surveillance: Early Case Detection
Potential sources of virus for a recurrence of person-to-person spread of SARS-CoV include reintroduction to humans from an animal reservoir, persistent infection in previously ill persons, or the laboratory. Since SARS-CoV currently exists in the animals in southern China -- the apparent source of the 2003 outbreak -- this area remains under scrutiny for SARS-CoV disease activity. Potential sources of recurrence also include other areas where SARS-CoV transmission occurred and large cities that are international travel hubs connecting to locales that might harbor persistent infections in humans. Laboratory personnel working with SARS-CoV might also become infected as a result of compromised laboratory techniques.1 Because persons with SARS-CoV disease tended to appear in clusters (e.g., in healthcare facilities, households, and a few special settings) during the 2003 outbreaks, early signals of the reappearance of the illness in U.S. communities could include unusual clusters of unexplained pneumonia.
In the absence of person-to-person transmission of SARS-CoV worldwide, the goal of domestic surveillance is to maximize early detection of cases of SARS-CoV disease while minimizing unnecessary laboratory testing, concerns about SARS-CoV, implementation of control measures, and social disruption. Early and efficient detection of SARS cases is not, however, a straightforward task. In the absence of known transmission worldwide, the overall likelihood that a person in the United States with fever and respiratory symptoms will have SARS-CoV disease is exceedingly low. Moreover, the non-specific clinical features of early SARS-CoV disease and the current lack of diagnostic tests that can reliably detect the virus during the first few days of illness pose challenges to finding SARS-CoV-infected persons during the predictable seasonal upsurge in respiratory infections.
Nonetheless, lessons learned from the 2003 outbreaks have identified three features of SARS-CoV disease that can be used to focus surveillance activities during the period of no transmission worldwide: 1) most patients infected with SARS-CoV develop radiographic evidence of pneumonia; 2) most SARS-CoV transmission occurs when patients are seriously ill and require hospitalization; and 3) most infected patients have an identifiable exposure to a known SARS-CoV case or a suggestive cluster of SARS-like illness or a location with known SARS transmission.
Given these features, the potential sources of recurrence of SARS-CoV, and the predilection for SARS-CoV transmission to occur in healthcare settings or to be associated with geographically focused pneumonia clusters, surveillance efforts in the absence of person-to-person SARS-CoV transmission should aim to identify patients who require hospitalization for radiographically confirmed pneumonia or acute respiratory distress syndrome without identifiable etiology AND who have one of the following risk factors in the 10 days before the onset of illness:
- Travel to mainland China, Hong Kong, or Taiwan, or close contact2 with an ill person with a history of recent travel to one of these areas, OR
- Employment in an occupation associated with a risk for SARS-CoV exposure (e.g., healthcare worker3 with direct patient contact; worker in a laboratory that contains live SARS-CoV), OR
- Part of a cluster of cases of atypical pneumonia without an alternative diagnosis
Infection control practitioners and other healthcare personnel should also be alert for clusters of pneumonia among two or more healthcare workers who work in the same facility.
The 2003 SARS-CoV outbreak likely originated in mainland China, and neighboring areas such as Taiwan and Hong Kong are thought to be at higher risk due to the large volume of travelers from mainland China. Although less likely, SARS-CoV may also reappear from other previously affected areas. Therefore, clinicians should obtain a complete travel history. If clinicians have concerns about the possibility of SARS-CoV disease in a patient with a history of travel to other previously affected areas (e.g., while traveling abroad, had close contact with another person with pneumonia of unknown etiology or spent time in a hospital in which patients with acute respiratory disease were treated), they should contact the health department.
In the absence of person-to-person transmission of SARS-CoV in the world, the screening of persons requiring hospitalization for radiographically confirmed pneumonia for risk factors suggesting SARS-CoV exposure should be limited to adults, unless there are special circumstances that make the clinician and public health personnel consider a child to be of potentially high risk for having SARS-CoV disease. During the 2003 global outbreaks, infants and children accounted for only a small percentage of SARS cases and had a much milder disease and better outcome than adults. Although information on SARS-CoV disease in pediatric patients is limited, the role of children in transmission is likely much less significant than the role of adults.
Case Detection
Severe respiratory illness in the context of a documented exposure risk is the key to diagnosing SARS-CoV disease. Providers should therefore consider SARS-CoV disease in patients requiring hospitalization for:
- Radiographically confirmed pneumonia or acute respiratory distress syndrome of unknown etiology, AND
- One of the following risk factors in the 10 days before illness onset:
- Travel to mainland China, Hong Kong, or Taiwan, or close contact with an ill person with a history of recent travel to one of these areas, OR
- Employment in an occupation associated with a risk for SARS-CoV exposure (e.g., healthcare worker with direct patient contact; worker in a laboratory that contains live SARS-CoV), OR
- Part of a cluster of cases of atypical pneumonia without an alternative diagnosis
Infection control practitioners and other healthcare personnel should be alert for clusters of pneumonia among two or more healthcare workers who work in the same facility.
Footnotes for Part III
- Persons who work in laboratories that contain live SARS-CoV should report any febrile and/or respiratory illnesses to the supervisor. They should be evaluated for possible exposures, and their clinical features and course of illness should be closely monitored, as described in Appendix F6, Supplement F, in Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS). If laboratory workers with fever and/or lower respiratory illness are found to have an exposure to SARS-CoV, they should be managed according to the algorithm in Figure 2 of Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness. In an exposed laboratory worker, symptoms that should trigger the clinical algorithm in Figure 2 should be expanded to include the presence of any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, diarrhea (see Figure 2, footnote 1, for more details).
- Close contact: A person who has cared for or lived with a person with SARS-CoV disease or had a high likelihood of direct contact with respiratory secretions and/or body fluids of a person with SARS-CoV disease. Examples of close contact include kissing or hugging, sharing eating or drinking utensils, talking within 3 feet, and direct touching. Close contact does not include activities such as walking by a person or briefly sitting across a waiting room or office.
- Healthcare worker: Any employee in a healthcare facility who has close contact with patients, patient-care areas, or patient-care items.
IV. Infection Control and Clinical Evaluation
SARS-CoV disease provides a reminder of the risks of nosocomial transmission of respiratory pathogens and an opportunity to improve overall infection control in healthcare facilities. All healthcare facilities need to re-emphasize the importance of basic infection control measures for the control of SARS-CoV disease and other respiratory illnesses. Facilities should also consider adopting a "respiratory hygiene/cough etiquette" strategy to help limit nosocomial transmission of respiratory pathogens. To contain respiratory secretions, all persons with signs and symptoms of a respiratory infection, regardless of presumed cause, should be instructed to:
- Cover the nose and mouth when coughing or sneezing
- Use tissues to contain respiratory secretions.
- Dispose of tissues in the nearest waste receptacle after use.
- Perform hand hygiene after contact with respiratory secretions and contaminated objects and materials.
Healthcare facilities should ensure the availability of materials for adhering to respiratory hygiene/cough etiquette in waiting areas for patients and visitors:
- Provide tissues and no-touch receptacles for used tissue disposal.
- Provide conveniently located dispensers for alcohol-based hand rug.
- Provide soap and disposable towels for hand washing where sinks are available.
During periods of increased respiratory infection in the community, healthcare facilities should offer procedure or surgical masks to persons who are coughing and encourage coughing persons to sit at least 3 feet away from others in waiting areas. Healthcare workers should practice Droplet Precautions, in addition to Standard Precautions, when examining a patient with symptoms of a respiratory infection. Droplet precautions should be maintained until it is determined that they are no longer needed. An algorithm for patient evaluation is provided in Appendix 1.
If the clinician and health department have a high index of suspicion for SARS-CoV disease or if laboratory evidence of SARS-CoV disease is found, then the patient should be placed immediately on SARS isolation precautions, and contacts should be immediately identified, evaluated, and monitored for evidence of respiratory disease. Prompt SARS-CoV laboratory diagnostics should be arranged through the health department. Initial diagnostic evaluation to look for an alternative diagnosis in suspected SARS-CoV patients should be performed as clinically indicated, and may include:
- Chest radiograph
- Pulse oximetry
- Complete blood count with differential
- Blood cultures
- Sputum Gram stain and culture
- Testing for viral respiratory pathogens, notably influenza A and B and respiratory syncytial virus
- Specimens for Legionella and pneumococcal urinary antigen testing
Infection Control and Clinical Evaluation
- Healthcare facilities should re-emphasize the importance of basic infection control measures for respiratory infections and consider adopting a "respiratory hygiene/cough etiquette" strategy.
- All patients admitted to the hospital with radiographically confirmed pneumonia should be:
- Placed on droplet precautions
- Screened for risk factors for possible exposure to SARS-CoV
- Evaluated with a chest radiograph, pulse oximetry, complete blood count, and etiologic workup as indicated.
- If there is a high index of suspicion for SARS-CoV disease (by clinicians and health department), the patient should immediately be placed on SARS isolation precautions, and all contacts of the ill patient should be identified, evaluated, and monitored. Prompt SARS-CoV laboratory diagnostics should be arranged through the health department.
V. Laboratory Testing for SARS-CoV
Laboratory testing for SARS-CoV is now available at many state public health laboratories. Available tests include antibody testing using an enzyme immunoassay (EIA) and reverse transcription polymerase chain reaction (RT-PCR) tests for respiratory, blood, and stool specimens. In the absence of person-to-person transmission of SARS-CoV, the positive predictive value of a diagnostic test is extremely low. False-positive test results may generate tremendous anxiety and concern and expend valuable public health resources. Therefore, SARS-CoV testing should be performed judiciously, and preferably only in consultation with the local or state health department. SARS-CoV testing should be considered if no alternative diagnosis is identified 72 hours after initiation of the clinical evaluation and the patient is thought to be at high risk for SARS-CoV disease (e.g., is part of a cluster of unexplained pneumonia cases).
Providers should immediately report all positive SARS-CoV test results to the local or state health department. Confirmatory SARS-CoV testing at an appropriate confirmatory test site should be arranged through the local or state health department as outlined in Supplement F, Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
Guidelines for the collection and transport of specimens for SARS-CoV testing are provided in Appendix F4, Supplement F, in Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
CDC is working with the Association of Public Health Laboratories (APHL) and the Laboratory Response Network (LRN) to ensure that SARS RT-PCR and EIA tests meet quality control guidelines. CDC will also be distributing proficiency testing materials to participating laboratories.
Laboratory testing for SARS-CoV
- Perform laboratory testing judiciously and in consultation with the local or state health department.
- Providers should report all positive test results immediately to the local or state health department.
- Arrange for confirmatory testing at an appropriate test site through the local or state health department.
VI. Reporting of Potential SARS-CoV Cases
Healthcare providers should report to the state or local health department:
- All persons requiring hospitalization for radiographically confirmed pneumonia who report at least one of the three risk factors for exposure to SARS-CoV outlined in Section III above.
- Any clusters (two or more persons) of unexplained pneumonia, especially among healthcare workers
- Any positive SARS-CoV test result
Note: Note: In the absence of known person-to-person transmission of SARS-CoV in the world, any SARS-CoV-positive test result should be phoned in to the state or local health department immediately for confirmation and implementation of urgent and appropriate isolation precautions, contact tracing, and follow-up.
Health departments should immediately report any SARS-CoV positive test result to CDC. Health departments should also inform CDC of other cases or clusters of pneumonia that are of particular concern by calling 770-488-7100.
Report to State or Local Health Department:
- All persons requiring hospitalization for radiographically confirmed pneumonia who report at least one of the three risk factors for exposure to SARS-CoV
- Any clusters of unexplained pneumonia, especially among healthcare workers
- Any positive SARS-CoV test result
Appendix 1
In the Absence of Person-to-Person Transmission of SARS-CoV Worldwide: Guidance for Evaluation and Management of Patients Requiring Hospitalization for Radiographically Confirmed Pneumonia
In the absence of SARS-CoV transmission in the world, a diagnosis of SARS-CoV disease should be considered only in patients who require hospitalization for radiographically confirmed pneumonia and who have an epidemiologic history that raises the suspicion for SARS-CoV disease. The suspicion for SARS-CoV disease is increased if, within 10 days of the onset of SARS-like symptoms, the patient: 1) traveled to mainland China, Hong Kong, or Taiwan, or had close contact with an ill person with a history of recent travel to one of these areas , 2) is employed in an occupation associated with a risk for SARS-CoV exposure (e.g., healthcare worker with direct patient contact; worker in a laboratory that contains live SARS-CoV), or 3) is part of a cluster of cases of atypical pneumonia without an alternative diagnosis. Persons with such a clinical and exposure history should be evaluated according to the following algorithm.
In some settings, early recognition of SARS-CoV disease may require additional measures:
Laboratory workers - Breaks in technique in laboratories that contain live SARS-CoV could result in laboratory-acquired cases of SARS. Persons working in laboratories that contain live SARS-CoV should report any fever and/or lower respiratory illness to the supervisor. They should be evaluated for possible exposures, and their clinical features and course of illness should be closely monitored as described in Appendix F6 in Supplement F, Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome (SARS).
If laboratory workers with fever and/or lower respiratory illness are found to have an exposure to SARS-CoV, they should be managed according to the algorithm in Figure 2 of Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness. In an exposed laboratory worker, symptoms that should trigger the clinical algorithm in Figure 2 should be expanded to include the presence of any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, diarrhea (see Figure 2, footnote 1, for more details).
Pediatric populations - Information on SARS-CoV disease in pediatric patients is limited. During the global outbreaks of 2003, infants and children accounted for only a small percentage of cases and had a much milder disease and better outcome than adults. Their role in transmission is not well described but is likely much less significant than the role of adults. Therefore, in the setting of no person-to-person SARS-CoV transmission in the world, the evaluation and management algorithm applies only to adults, unless there are special circumstances that make the clinical and health department consider a child to be of potentially high risk for having SARS-CoV disease.
Figure 1. Algorithm for evaluation and management of patients requiring hospitalization for radiographically confirmed pneumonia, in the absence of person-to-person transmission of SARS-CoV in the world
Top of PageAppendix 2
Guidelines for Collecting Specimens from Potential SARS Patients
This document updates and replaces the guidelines for specimen collection posted previously on CDC’s SARS website, to reflect the most recent information on laboratory diagnostics for SARS-CoV. The main changes are as follows:1
- Addition of stool, serum, and plasma to the list of specimens for RT-PCR testing
- Addition of information on the optimal timing of specimen collection and testing by specimen type
- Recommendation to collect multiple specimens for RT-PCR testing
Footnotes for Appendix 2
- Pending IRB approval.
Key Messages
- Consult the state or local health department to determine the appropriateness and details of SARS testing.
- If possible, collect multiple specimens from different body sites and at different times during illness.
- A signed consent form is recommended when collecting specimens for SARS-CoV RT-PCR or antibody testing.
Before collecting and shipping specimens for SARS-CoV testing, consult with the state health department/state epidemiologist to determine whether the patient meets the SARS case definition. Health department contact information is available at: http://www.cste.org/?page=POCOverview.
When possible, collect multiple respiratory specimens for testing. For example, collect specimens from two different sites on the same day (e.g., one nasopharyngeal swab and a stool specimen or another respiratory specimen) or from two different times during the illness. The chart on the last page specifies recommended specimen types for SARS-CoV diagnostics by stage of illness, including postmortem specimen collection.
A signed consent form is recommended when collecting specimens for SARS-CoV RT-PCR or antibody testing.
RT-PCR Diagnostics
Although studies to date have not definitively determined the best specimens for SARS RT-PCR diagnostics, it is reasonable to collect:
- During the first week of illness: Nasophyaryngeal (NP) swab plus oropharygeal (OP) swab and a serum or plasma specimen
- After the first week of illness: NP swab plus OP swab and a stool specimen
Serologic Diagnostics
Serum specimens for SARS-CoV antibody testing should be collected when the diagnosis is first suspected and at later times if indicated. An antibody response is occasionally detected during the first week of illness, likely to be detected by the end of the second week of illness, and sometimes may not be detected until > 28 days after onset of symptoms.
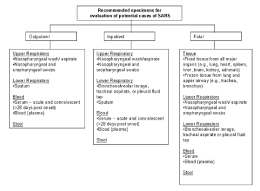
I. Collecting Respiratory Specimens
Eight types of respiratory specimens may be collected for viral and/or bacterial diagnostics: 1) nasopharyngeal wash/aspirates, 2) nasopharyngeal swabs, 3) oropharyngeal swabs, 4) broncheoalveolar lavage, 5) tracheal aspirate, 6) pleural fluid tap, 7) sputum; and 8) post-mortem tissue. Nasopharyngeal wash/aspirates are the specimen of choice for detection of most respiratory viruses and are the preferred specimen type for children under age 2 years.
In contrast to most respiratory pathogens for which respiratory specimens are optimally collected within 72 hours after the onset of symptoms, levels of SARS-CoV may be higher later in the course of the illness.
Before collecting specimens, review the infection control precautions in Supplement I.
A. Collecting specimens from the upper respiratory tract
- Nasopharyngeal wash/aspirate
Have the patient sit with head tilted slightly backward. Instill 1 ml-1.5 ml of nonbacteriostatic saline (pH 7.0) into one nostril. Flush a plastic catheter or tubing with 2 ml-3 ml of saline. Insert the tubing into the nostril parallel to the palate. Aspirate nasopharyngeal secretions. Repeat this procedure for the other nostril. Collect the specimens in sterile vials. Label each specimen container with the patient's ID number and the date collected. If shipping domestically, use cold packs to keep the sample at 4°C. If shipping internationally, pack in dry ice. - Nasopharyngeal or oropharyngeal swabs
Use only sterile dacron or rayon swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden sticks, as they may contain substances that inactivate some viruses and inhibit PCR testing.- Nasopharyngeal swabs -- Insert a swab into the nostril parallel to the palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nostrils.
- Oropharyngeal swabs -- Swab the posterior pharynx and tonsillar areas, avoiding the tongue.
Place the swabs immediately into sterile vials containing 2 ml of viral transport media. Break the applicator sticks off near the tip to permit tightening of the cap. Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
B. Collecting specimens from the lower respiratory tract
- Broncheoalveolar lavage, tracheal aspirate, pleural fluid tap
Centrifuge half of the specimen, and fix the cell pellet in formalin. Place the remaining unspun fluid in sterile vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ®. Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, ship fixed cells at room temperature and unfixed cells frozen. - Sputum
Educate the patient about the difference between sputum and oral secretions. Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile screw-cap sputum collection cup or sterile dry container. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
II. Collecting Blood Components
Serum and blood (plasma) should be collected early in the illness for RT-PCR testing. The reliability of RT-PCR testing performed on blood specimens decreases as the illness progresses.
Both acute and convalescent serum specimens should be collected for antibody testing. To confirm or rule out SARS-CoV infection, it is important to collect convalescent serum specimens >28 days after the onset of illness.
A. Collecting serum for antibody or RT-PCR testing
Collect 5 ml-10 ml of whole blood in a serum separator tube. Allow the blood to clot, centrifuge briefly, and collect all resulting sera in vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm®. The minimum amount of serum preferred for each test is 200 microliters, which can easily be obtained from 5 mL of whole blood.
A minimum of 1 cc of whole blood is needed for testing of pediatric patients. If possible, collect 1 cc in an EDTA tube and in a clotting tube. If only 1cc can be obtained, use a clotting tube.
Label each specimen container with the patient's ID number and the date the specimen was collected. If unfrozen and transported domestically, ship with cold packs to keep the sample at 4°C. If frozen or transported internationally, ship on dry ice.
B. Collecting EDTA blood (plasma) for RT-PCR
Collect 5 ml-10 ml of blood in an EDTA (purple-top) tube. Transfer to vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ®. Label each specimen container with patient's ID number and date of collection. Store and ship blood specimens with cold packs to keep the sample at 4°C.
III. Collecting Stool Specimens for PCR
Begin collecting stool specimens as soon as possible in the course of the illness. Although collecting earlier specimens is ideal, SARS-CoV has been detected in stool as late as one month after the onset of symptoms.
Place each stool specimen - as large a quantity as can be obtained (at least 10 cc) - in a leak-proof, clean, dry container, and refrigerate at 4°C. Patients may drape plastic kitchen wrap across the back half of the toilet, under the toilet seat, to facilitate collection of stool specimens.
IMPORTANT: Refrigerate or freeze tubes after specimens are placed in them. If specimens will be examined within 48 hours after collection, they can be refrigerated. If specimens must be held longer than 48 hours, freeze them as soon as possible after collection. Although storage in an ultra-low freezer (-70°C) is preferable, storage in a home-type freezer (if properly set at -20°C) is acceptable for short periods.
Specimens from possible and known SARS cases must be packaged, shipped, and transported according to the current edition of the "International Air Transport Association (IATA) Dangerous Goods Regulations" and US DOT 49 CFR Parts 171-180 at phmsa.dot.gov/hazmat.
See step-by-step instructions on appropriate packaging and labeling.
Specimens for SARS-CoV Testing: Priority Specimens and Timing for Collection
The likelihood of detecting infection is increased if multiple specimens, e.g., stool, serum, and respiratory tract specimens, are collected during the course of illness.
| Specimen, by test type | <1 week after symptom onset | 1-3 weeks after symptom onset | >3 weeks after symptom onset | |
|---|---|---|---|---|
| RT-PCR 1 for viral RNA detection | Sputum 2 |
|
| |
| Bronchoalveolar lavage, tracheal aspirate, or pleural fluid tap 4 |
|
|
| |
| Nasopharyngeal wash/aspirate |
|
|
| |
| Nasopharyngeal and oropharyngeal swabs |
|
|
| |
| Serum (serum separator tube) |
|
| not recommended | |
Blood (plasma) (EDTA/purple top tube for plasma) |
|
| not recommended | |
| Stool (minimum 10 cc specimen) |
|
|
| |
| EIA 1 for antibody detection | Serum 5 (serum separator tube) |
|
|
|
Footnotes for Appendix 2 Part III
- Because of the investigational nature of both the SARS RT-PCR (reverse transcription-polymerase chain reaction) and the SARS EIA (enzyme immunoassay), it is recommended that the clinician obtain a signed informed consent form from the patient. The consent form for the RT-PCR test can be found online. The consent form for the EIA test can also be found online.
- A sputum specimen is preferred if the patient has a productive cough.
- The more checks, the better the results from a particular specimen at a specific point in the illness.
- Consider these specimen types if sputum is not available.
- Also collect a convalescent specimen >28 days post onset.
Related Pages
- Page last reviewed: May 3, 2005
- Page last updated: May 3, 2005
- Content source:


 ShareCompartir
ShareCompartir