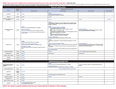
Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger, UNITED STATES, 2017
United States, 2017
Details for Healthcare Professionals
Each year, the Advisory Committee on Immunization Practices (ACIP) approves immunization schedules for persons living in the United States. The immunization schedule for children and adolescents aged 18 years or younger provides a summary of ACIP recommendations on the use of routinely recommended vaccines. The immunization schedule as well as the catch-up immunization schedule have also been approved by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG).
2017 Immunization Schedule
In October 2016, ACIP approved the 2017 Immunization Schedule for children and adolescents birth through 18 years, effective February 1, 2017.
The comprehensive summary of the ACIP recommended changes made to the schedule can be found in the MMWR.
Changes to the schedule include:
Hepatitis B vaccine (Updated 3/6/2017)
- Monovalent Hepatitis B vaccine should be administered within 24h of birth for medically stable infants weighing ≥2,000 grams born to hepatitis B surface antigen (HBsAg)-negative mothers. The recommendations for vaccination of infants <2,000 grams (as well as infants born to HBsAg-positive mothers or mothers whose hepatitis B status is unknown) remain unchanged.
- Preterm infants weighing <2,000 g born to HBsAg-negative mothers should receive the first dose of vaccine 1 month after birth or at hospital discharge.
- Additional detail regarding Hepatitis B vaccination of infants born to HBsAg-positive mothers or mothers whose hepatitis B status is unknown can be found in the ACIP Hepatitis B recommendations [39 pages].
Polio vaccine (New 2/17/2017)
The January 13 and February 17, 2017 MMWRs provide additional guidance regarding assessment of poliovirus vaccination status and vaccination of children who have received poliovirus vaccine outside the United States. View the complete guidance.
Specifically, the guidance provides information which offers additional clarification and changes impacting the IPV footnote in the 2017 catch-up schedule:
- If both OPV and IPV were administered as part of a series, the total number of doses needed to complete the series is the same as that recommended for the U.S. IPV schedule. A minimum interval of 4 weeks should separate doses in the series, with the final dose administered on or after the fourth birthday and at least 6 months after the previous dose.
- If only OPV was administered, and all doses were given before age 4 years, 1 dose of IPV should be given at 4 years or older and at least 6 months after the last OPV dose. This 6-month period is a change from the current footnote which indicates the period is 4 weeks after the last OPV dose.
- Only trivalent OPV (tOPV) counts toward the U.S. vaccination requirements (tOPV was used for routine poliovirus vaccination in all OPV-using countries until April 1, 2016). See Guidance to assess doses documented as “OPV”.
Medical Conditions
A new figure, Figure 3 “Vaccines that might be indicated for children and adolescents aged 18 years or younger based on medical indications”, has been added. This figure:
- Demonstrates most children with medical conditions can (and should) be vaccinated according to the routine child/adolescent immunization schedule.
- Indicates when a medical condition is a precaution or contraindication to vaccination.
- Indicates when additional doses of vaccines may be necessary because of a child’s or adolescent’s medical condition. Providers should consult the relevant footnotes for additional information.
Diphtheria and tetanus toxoids and acellular pertussis vaccine
- The diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) footnote was revised to more clearly present recommendations following an inadvertently early administered fourth dose of DTaP.
- The tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap) footnote for vaccination of pregnant adolescents between gestational weeks 27–36 has been updated to reflect a preference for vaccination earlier during this period. Currently available data suggest that vaccinating earlier in the 27 through 36 week time period will maximize passive antibody transfer to the infant.
Haemophilus influenzae type B vaccine
- Within the Haemophilus influenzae type b vaccine (Hib) footnote, Comvax was removed from the routine vaccination portion of footnote. This vaccine has been removed from the market, and all available doses have expired. Additionally, Hiberix has been added to the list of vaccines that may be used for the primary vaccination series.
Human papillomavirus vaccine
- A blue bar was added to the schedule for human papillomavirus vaccine (HPV) for children aged 9–10 years, indicating that persons in this age group may be vaccinated (even in the absence of a high-risk condition).
- The footnote for HPV vaccine has been updated to include the new 2-dose schedule for persons initiating the HPV vaccination series before age 15 years. Additionally, bivalent HPV vaccine has been removed from the schedule. This vaccine has been removed from the U.S. market, and all available vaccine doses have expired
Influenza vaccine
- Live attenuated influenza vaccine (LAIV) has been removed from the influenza row of the schedule.
- The influenza vaccine footnote has been updated to indicate that LAIV should not be used during the 2016-2017 influenza season.
Meningococcal vaccine
- The 16-year age column of the schedule has been separated from the 17–18-year age column to highlight the need for a meningococcal conjugate vaccine booster dose at age 16 years.
- The meningococcal vaccines footnote has been updated to include recommendations for meningococcal vaccination of children with human immunodeficiency virus (HIV) infection and to reflect recommendations for the use of a 2-dose Trumenba (meningococcal B vaccine) schedule.
Pneumococcal vaccine
- Within the pneumococcal vaccine footnote, references to 7-valent pneumococcal conjugate vaccine (PCV7) have been removed. All healthy children who may have received PCV7 as part of a primary series have now aged out of the recommendation for pneumococcal vaccine.
View and Print Schedules
Combined Schedule Format

Birth-18 Years and Catch-up
- Print combined schedules (including intro and footnotes)
- Order free copies from CDC (See note for instructions)
- Purchase laminated copies from Immunization Action Coalition (IAC)
Single Page Formats
Birth-18 Years Recommended Immunization Schedule
Print the full immunization schedule or display it on your website.
- Print and view schedule
-
Display schedule on your website
This method ensures schedules on your site will always be the most current; whenever CDC updates a schedule, your page will automatically display the same update. Visitors can print the schedule from your web page.
Child and Adolescent Immunization Schedule by Medical and Other Indications
Display and print this schedule on your website.
- Print and view schedule
-
Display schedule on your website
This method ensures schedules on your site will always be the most current; whenever CDC updates a schedule, your page will automatically display the same update. Visitors can print the schedule from your web page.
Catch-up Immunization Schedule
Display and print this schedule on your website.
- Print and view catch-up schedule
-
Display schedule on your website
This method ensures schedules on your site will always be the most current; whenever CDC updates a schedule, your page will automatically display the same update. Visitors can print the schedule from your web page. - Order free copies from CDC (see note for instructions)
CDC has developed job aids to assist healthcare providers in interpreting figure 2 in the childhood/adolescent immunization catch-up schedule.
Laminated Pocket-size Schedules
The childhood/adolescent immunization schedule is available in a smaller, portable format and includes the catch-up schedule and footnotes. Order free copies from CDC (See note for instructions)
Easy-to-read Versions for Parents

Interactive Tools
Get the CDC Vaccine Schedules App
Note: If you previously downloaded the tool, check that you have version 4.0.1 with 2017 schedules and footnotes.
Create a Schedule of Vaccines A Child May Need
Birth Through 6 Years
Create a Customized Schedule of Vaccines Needed (birth to 6 years)
Use this tool to create a personalized schedule you can give to and discuss with parents. Parents also can use this print-out to record their child’s vaccinations. Enter the patient’s date of birth, click “get schedule”, and then click “printable schedule.”
Generate a Catch-up or Accelerated Schedule
Birth Through 18 Years
Use this tool to generate a catch-up or accelerated schedule (ages birth through 18). This Catch-up Schedule will identify doses and timing of vaccines needed (useful when a child needs to travel, must catch-up on missed doses, or during a disease outbreak).
Take a Quiz to See Vaccines A Child May Need
Birth Through 18 Years
Take this Childhood Vaccine Quiz to create a personalized list of recommended vaccines for a child (ages birth through 18) based on his or her health history. Once printed, discuss with parents.
Binational Resource

Presentation Graphics
Download the adult immunization schedule as a PowerPoint presentation
Immunization Recommendations
Catch-Up Guidance Job Aids
CDC has developed job aids to assist healthcare providers in interpreting figure 2 in the Childhood/Adolescent immunization catch-up schedule.
- Pneumococcal Conjugate Vaccine (PCV) Catch-Up Guidance for Children 4 Months through 18 Years of Age [3 pages]
- Haemophilus Influenzae type b-Containing Vaccines Catch-Up Guidance for Children 4 Months through 18 Years of Age
- Diphtheria, Tetanus, and Pertussis-Containing Vaccines Catch-Up Guidance for Children 4 Months through 18 Years of Age [4 pages]
Related Pages
- Past Immunization Schedules
- Provider Resources for Vaccine Conversations with Parents
- Vaccination Records for Kids
- Vaccine administration record for children and teens [6 pages]
- Screening Questionnaire for Child and Teen Immunization [2 pages]
- Interpreting abbreviations on records
- Standards for immunization practices for children and adolescents
- Vaccines in delay or shortage
- Notification of Vaccination Letter Template [1 page]
Note: To order the Child and Adolescent immunization schedule, go to CDC-INFO on Demand – Publications and pull down the menu for “Programs”. Select “Immunization and Vaccines (Schedules and Booklets)” and click “Search”.
- Page last reviewed: February 6, 2017
- Page last updated: October 5, 2017
- Content source:


 ShareCompartir
ShareCompartir