We need you! Join our contributor community and become a WikEM editor through our open and transparent promotion process.
Smallpox
From WikEM
(Redirected from Variola)
Contents
Background
- Caused by the variola virus[1]
- Passed through direct contact with the person, with body fluids, as well as with airborne droplets of an infected, symptomatic person
- Most infectious during the first week of symptoms, but will remain infectious until the last pox scab falls off
- Humans are the only known host[2]
- Declared eradicated in 1980 after a global immunization campaign from 1966-1980; last known natural case was in Somalia in 1977; last known cases were in England in 1978 after a laboratory accident[3]
Bioterrorism
- There are stocks of the virus in 2 laboratories – one in Atlanta, Georgia, USA and one in Moscow, Russia
- There are concerns some laboratories may illegally have the virus and could release it as a weapon of bioterrorism[4]
Vaccination History
- The vaccine “vaccinia variola” was made from a closely-related virus
- Vaccination is considered successful if at least one pustule forms at the injection site
- Does have serious side effects, especially in the immunocompromised, including death in rare cases
- Due to the side effects and the current eradication, it is not used anywhere in the world currently
- Many governments have large stockpiles of the vaccinia vaccine and plans in place for rapid response and vaccination if an outbreak were to occur
Clinical Features
- Incubation period: 7-19 days
- Initial phase begins as a fever, fatigue/weakness, dorsal-lumbar pain, myalgias, nausea/vomiting
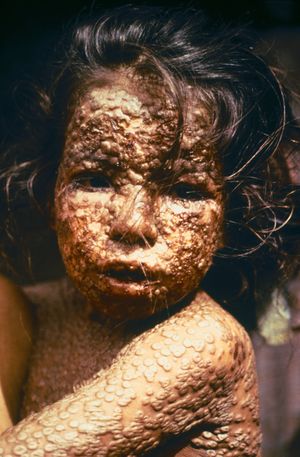
- 2-4 days later the characteristic rash appears
- Worst on the face, arms, legs, and includes the palms and soles
- Lesions will generally all be at same stage
- Lesions begin as clear fluid-filled vesicles, progress to pustules, and then harden and form a crust, ultimately falling off in about 3-4 weeks
Disease courses
- Variola minor – most common form of the disease, described above
- Variola fulminans – rapid death during the initial phase
- Variola confluens – initial maculopapular rash becomes confluent leading to 96% mortality
- Variola hemorrhagica – hemorrhages occur within the blisters as well as mucus membranes and internal organs, death usually occurs during the first 24 hours
Differential Diagnosis
Pediatric Rash
- Drug rash
- Erythema Infectiosum (Fifth disease)
- Hand-foot-and-mouth disease
- Henoch-Schonlein Purpura (HSP)
- Herpangina
- Herpes simplex virus
- Infectious Mononucleosis
- Meningitis
- Measles
- Molluscum contagiosum
- Roseola infantum
- Rubella German measles)
- Scarlet fever
- Smallpox
- Varicella (Chickenpox)
Vesiculobullous rashes
Febrile
- Diffuse distribution
- Varicella
- Smallpox
- Disseminated gonococcal disease
- DIC
- Purpural fulminans
- Localized distribution
Afebrile
- Diffuse distribution
- Bullous pemphigoid
- Drug-Induced bullous disorders
- Pemphigus vulgaris
- Phytophotodermatitis
- Erythema multiforme major
- Localized distribution
Bioterrorism Agents[5]
Category A
Category B
- Ricin
- Brucellosis
- Epsilon toxin
- Psittacosis
- Q Fever
- Staph enterotoxin B
- Typhus
- Glanders
- Melioidosis
- Food safety threats
- Water safety threats
- Viral encephalitis
Category C
- Influenza
- Yellow fever
- Tickborne hemorrhagic fever
- Tickborne encephalitis
Evaluation
- Clinical diagnosis based on symptoms and characteristic rash
- PCR DNA test
- When the disease was present, either electron microscopy of stained crusts of lesions or a slide precipitation method was used
Management
- IMMEDIATE NOTIFICATION OF PUBLIC HEALTH AUTHORITIES
- Vaccine administered up to 3 days post-exposure was effective in preventing infection as well as lessening the severity of the disease if infection occurred [6]
Postexposure Prophylaxis
- Vaccinia Vaccine (administer within 72hrs of exposure)
Active Disease
- Vaccinia Vaccine within the first 72hrs can decrease total disease severity and within 7 days may decrease symptoms
- Supportive care and wound care for open lesions
Vaccinia Vaccine Complications
Contraindications for administration include:
- Pregnancy
- Severe cardiac disease
- Immunocompromise
- Same living quarters as other person with above contraindications
- due to viral shedding
Isolation
- Airborne and contact isolation with negative pressure
- Personal protective wear level D with N95 respirator
See Also
References
- ↑ Rosen, Peter, John A. Marx, Robert S. Hockberger, and Ron M. Walls. "Smallpox." Rosen's Emergency Medicine Concepts and Clinical Practice. 8th ed. Vol. 2. Philadelphia, PA: Elsevier/Saunders, 2013. 1579-1580.
- ↑ Barquet, Nicolau, MD, and Pere Domingo, MD. "Smallpox: The Triumph over the Most Terrible of the Ministers of Death." Annals of Internal Medicine 127 (1997): 635-42.
- ↑ Ellner, P. D. "Smallpox: Gone but Not Forgotten." Infection 26.5 (1998): 263-69.
- ↑ Anderson PD. Bokor G. Bioterrorism: pathogens as weapons. J Pharm Pract. 2012 Oct;25(5):521-9.
- ↑ https://www.niaid.nih.gov/topics/biodefenserelated/biodefense/pages/cata.aspx Accessed 02/26/16
- ↑ Kman NE, Nelson RN. Infectious agents of bioterrorism: a review for emergency physicians. Emerg Med Clin North Am. 2008 May;26(2):517-47