Decoding Middle East Respiratory Syndrome Coronavirus: AMD provides quick answers
The AMD program, which began in 2014, represents a significant leap forward for CDC’s advanced sequencing and bioinformatics capabilities. Prior to receiving AMD funding, CDC incorporated genetic sequencing methods in some infectious disease outbreak responses. This “AMD in Action” story about CDC’s pre-AMD funded work foreshadows the more robust and far-reaching effort launched in 2014—an escalated attack on infectious disease outbreaks that is proving to be better, quicker, and cheaper.
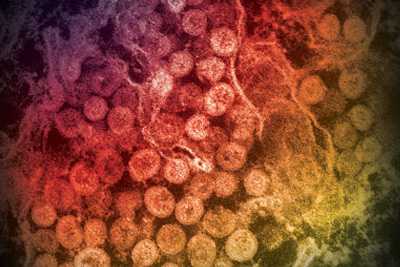
An electron micrograph showing spherical particles within the cytoplasm of an infected cell.
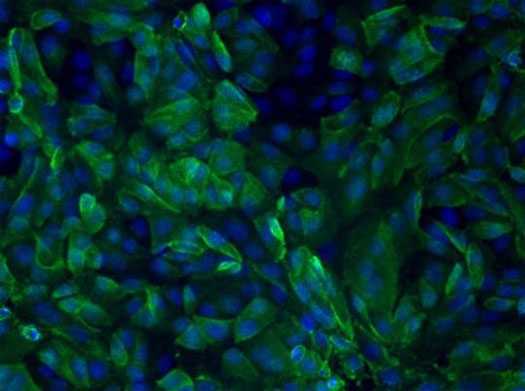
Human serum antibodies react with MERS-CoV-infected Vero cells, indicating the patient has been infected with MERS-CoV. (Image source: Jennifer L. Harcourt, CDC).
The first case of Middle East Respiratory Syndrome (MERS) in the United States was confirmed in a traveler to Indiana from Saudi Arabia in early May 2014. On May 1, 2014, the Indiana State Department of Health obtained an initial positive laboratory result for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) from a serum specimen from the patient. CDC confirmed the positive result on May 2, within hours of receiving the specimen.
On May 3, CDC received an additional sputum (mucus coughed up from the lower airways) specimen from the patient. In less than 48 hours, CDC used Advanced Molecular Detection (AMD) methods to sequence the complete virus genome.
Just before this first case of MERS surfaced in the United States, the number of cases reported from Saudi Arabia had more than doubled from the previous year. Public health investigators were concerned that changes in the virus could be spurring the increase. Major changes in the virus might allow MERS-CoV to spread more easily from person to person. Combining disease tracking information with sequencing technology helps public health investigators understand more about the virus and how it spreads. And better understanding may lead to stronger safeguards for the nation's health.
AMD methods showed that this sequence was similar to other known MERS-CoV sequences. The rapid pace with which this new sequence information was available and parallel work reported from Germany allowed CDC to sooth concerns about significant changes in the virus. Work has already started on ways to improve the efficiency and timeliness of this type of result. These methods are also applicable to unknown agents and other emergent threats.
In addition, CDC sent the sequence to GenBank to make it available to the scientific community for further testing and analysis, which will increase knowledge about the virus and how to combat it.
- Page last reviewed: August 17, 2015
- Page last updated: August 17, 2015
- Content source:
- Centers for Disease Control and Prevention
- Page maintained by: Office of Associate Director of Communication, Division of Public Affairs


 ShareCompartir
ShareCompartir