Global Health Security Agenda: GHSA Real-Time Surveillance Action Package (GHSA Action Package Detect-2/3)
 Five-Year Target: Strengthened foundational indicator- and event-based surveillance systems that are able to detect events of significance for public health, animal health and health security; improved communication and collaboration across sectors and between sub-national, national and international levels of authority regarding surveillance of events of public health significance; improved country and regional capacity to analyze and link data from and between strengthened, real-time surveillance systems, including interoperable, interconnected electronic reporting systems. This can include epidemiologic, clinical, laboratory, environmental testing, product safety and quality, and bioinformatics data; and advancement in fulfilling the core capacity requirements for surveillance in accordance with the IHR and the OIE standards.
Five-Year Target: Strengthened foundational indicator- and event-based surveillance systems that are able to detect events of significance for public health, animal health and health security; improved communication and collaboration across sectors and between sub-national, national and international levels of authority regarding surveillance of events of public health significance; improved country and regional capacity to analyze and link data from and between strengthened, real-time surveillance systems, including interoperable, interconnected electronic reporting systems. This can include epidemiologic, clinical, laboratory, environmental testing, product safety and quality, and bioinformatics data; and advancement in fulfilling the core capacity requirements for surveillance in accordance with the IHR and the OIE standards.
As Measured by: Surveillance for at least three core syndromes6 indicative of potential public health emergencies conducted according to international standards.
Desired National Impact: A functioning public health surveillance system capable of identifying potential events of concern for public health and health security, and country and regional capacity to analyze and link data from and between strengthened real-time surveillance systems, including interoperable, interconnected electronic reporting systems. Countries will support the use of interoperable, interconnected systems capable of linking and integrating multi-sectoral surveillance data and using resulting information to enhance the capacity to quickly detect and respond to developing biological threats. Foundational capacity is necessary for both indicator-based (including syndromic) surveillance and event-based surveillance, in order to support prevention and control activities and intervention targeting for both established infectious diseases and new and emerging public health threats. Strong surveillance will support the timely recognition of the emergence of relatively rare or previously undescribed pathogens in specific countries.
Country Commitments to Action Package:
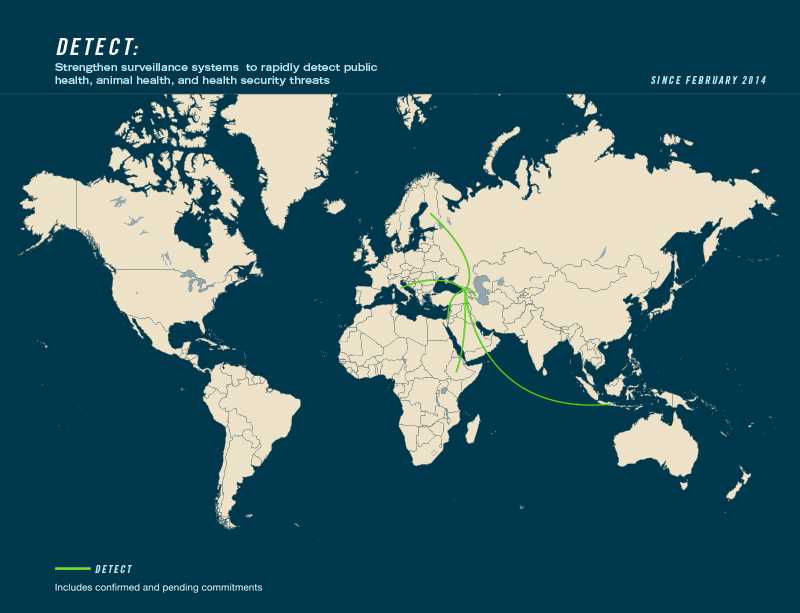
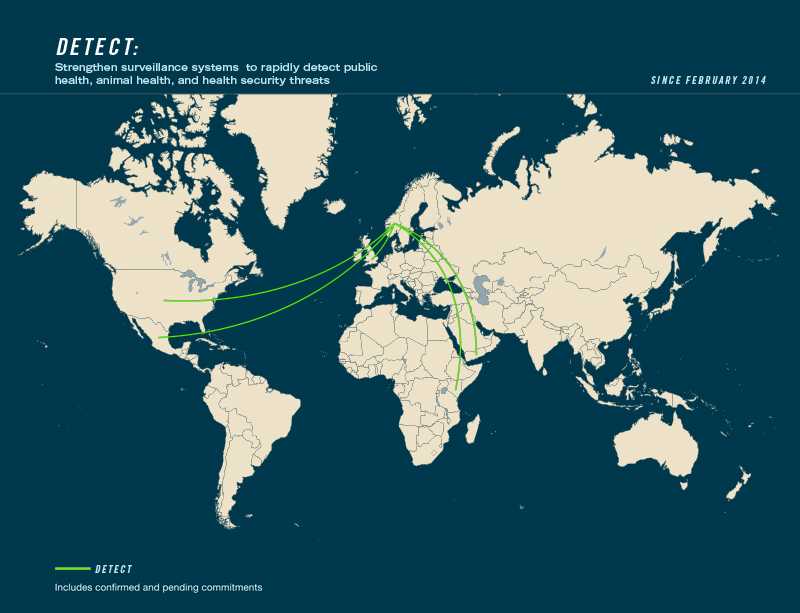
- Leading countries: Georgia, Norway
- Contributing countries: Azerbaijan, Ethiopia, Finland, Indonesia, Israel, Italy, Kenya, Mexico, United Kingdom, United States, Yemen
- Contributing international organizations: FAO, OIE, WHO
Five-Year Action Items:
Actions will be coordinated, as appropriate, with relevant international organizations including FAO, OIE and WHO.
- Collaborate with partner countries, FAO, OIE, WHO and other relevant partner organizations to adopt and implement agreed upon standards for surveillance data.
- Accelerate improvement and implementation of syndrome- and/or event-based surveillance systems, including those related to food and drug safety e.g. through the IDSR framework and guidelines or similar existing programs. Such systems will include opportunities for international or multi-sectoral cooperation.
- Establish or improve lines of communication with public health laboratories, including reference laboratories at the national and international levels.
- Establish links to an international reference network for the identification and verification of emerging pathogens that cannot be identified within the country.
- Strengthen or, if needed, develop surveillance systems that reach across all sectors of government to transmit standardized electronic surveillance data from regional, district, and community levels to a central national hub.
- Develop the appropriate technical mechanisms to integrate the surveillance data feeds from different sectors for expert analysis and interpretation to benefit decision-makers at relevant levels of government and/or a central national hub.
- Coordinate event-based surveillance with existing reference microbial laboratory networks as well as WHO IHR notification systems and national focal points, and through notification of OIE listed diseases.
- Sustain indicator-based surveillance, including syndromic surveillance, and event-based surveillance through training workshops, development of guidelines, provision of expert trainers, materials, and curricula, or other contributions in kind.
- Contribute to an international framework document for assessment of surveillance systems.
- Share lessons learned and pool resources on a regional basis for more efficient reporting, investigation and response.
- Support other countries in the establishment and/or strengthening of national public health surveillance systems as requested by the IHR paragraph 44 and consistent with Article X of the Biological and Toxin Weapons Convention.
- Collaborate with FAO, OIE and WHO to harmonize standards of reporting, quality of data, and IHR compliance.
Baseline Assessment and Planning Activities
- Convene a technical discussion with FAO, OIE, WHO and partner countries to develop and implement minimum standards for surveillance data, and review existing tools and systems for surveillance, and promote a common understanding, integration, and interoperability among all relevant sectors of government.
- Identify gaps in surveillance and diagnostic capacity and develop a national strategy for addressing identified weaknesses.
- Identify and promote information technology solutions in countries and across regions that strengthen routine and event-based surveillance through timely and accurate data capture, dissemination, and response. Create an inventory of current information technology (IT) systems and work to ensure interoperability among them, ensuring that parallel electronic systems are not created.
- Determine the prevalence of the 3 core pathogens and of those other pathogens that present as similar syndromes (e.g., acute nonspecific febrile illness) in each major geographical region of the country.
- Define the epidemiologic objectives/targets for surveillance, develop guidelines and job aids, and train health professionals prior to introducing or amending IT activities.
- Identify opportunities to utilize existing surveillance guidelines such as the IDSR framework and the WHO’s guidelines for implementation of early warning and response.
- Perform risk assessment.
Monitoring and Evaluation Activities
- Evaluate effectiveness of surveillance system enhancements.
6 Internationally recognized standards for syndromic surveillance are available for the following five syndromes: severe acute respiratory syndrome, acute flaccid paralysis, acute hemorrhagic fever, acute watery diarrhea with dehydration, and jaundice with fever. The three syndromes chosen will depend on national disease control priorities. These surveillance systems should include early warning surveillance data and laboratory findings, which should be analyzed by trained epidemiologists (see Ijaz et al., “What gets measured gets done. Emerging Infectious Diseases July 2012; 18:1054-7).
- Page last reviewed: December 24, 2014
- Page last updated: December 24, 2014
- Content source:
Global Health
Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.


 ShareCompartir
ShareCompartir