HoSt/VDSCP: Standardization of Measurement Procedures
The CDC Laboratory/Manufacturer Hormone Standardization (HoSt) Program and the Vitamin D Standardization Certification Program(VDSCP) help laboratories with lab-developed tests and assay manufacturers with calibration. This program assesses the assay performance (bias and imprecision) of the lab or assay manufacturer throughout 1 year.
The program consists of two phases:
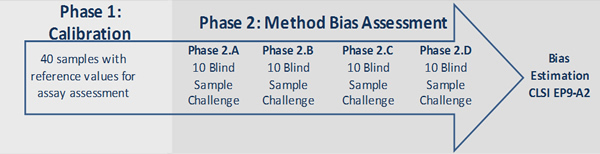
Phase 1
The participant receives 40 serum samples with reference value assignments. The serum samples are nonpooled, single donor sera prepared following CLSI protocol C37 (3) from male and female donors. These samples are intended to assess the accuracy of the test and to help in recalibrating the test as needed. Participants who are unable to fully participate in HoSt may obtain the 40 serum sample panel for method evaluation purposes.
Phase 2
After calibration, the participant ships four quarterly challenges (Phase 2A-D) with unknown serum samples for analysis. Values reported from participants on these serum samples are used for bias assessment as described in CLSI guidelines EP9-A2 (4) “Method Comparison and Bias Estimation using Patient Samples.” Current acceptable criteria are derived from data on published biological variability. CDC will consider participants meeting the criteria standardized and will list them on this Web site.
CDC HoSt Program and VDSCP provide preliminary reports during the quarterly challenges and technical assistance to resolve potential problems, thus ensuring the participant’s long-term success in maintaining standardized measurements.
All participants will remain anonymous. Only on successful completion of the CDC HoStProgram or VDSCP will CDC mention participation publicly.
Schematic of the HoSt and VDSCP
The CDC HoSt Testosterone program started in January 2010.
- HoSt – Standardization of Serum Total Testosterone Measurement Protocol [PDF – 668 KB] – Updated March 2014
- HoSt Testosterone Certified Procedures [PDF – 120 KB] – Updated June 2017
The CDC VDSCP program for total 25-hydroxyvitamin D (25OHD) started in December 2012.
- VDSCP – Standardization Certification Program Protocol for Total 25-hydroxyvitamin D Measurement [PDF – 661 KB] – Updated March 2014
- VDSCP Vitamin D Certified Procedures [PDF – 238 KB] – Updated June 2017
Phase 2 of the CDC HoSt Estradiol (E2) program started in 2014.
- HoSt – Standardization of Serum Total Estradiol Measurement Protocol [PDF – 562 KB] – Updated March 2014
- HoSt Estradiol Certified Procedures [PDF – 136 KB] – Updated June 2017
If you are interested participating in the CDC Hormone Standardization Program (HoSt) or the Vitamin D Standardization Certification Program(VDSCP), please complete the CDC Standardization Program Information Request Form [PDF – 335 KB] and return it to Standardization@cdc.gov.
- Page last reviewed: July 6, 2017
- Page last updated: July 6, 2017
- Content source:


 ShareCompartir
ShareCompartir