 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pelvic Inflammatory Disease: Guidelines for Prevention and ManagementBrief Summary This report provides comprehensive guidelines to aid practitioners and decision makers in achieving PID prevention and management objectives. The main focus of this document is PID related to STD. These guidelines for the prevention and management of PID were established by staff of CDC in consultation with a group of outside experts. Current data regarding the efficacy of prevention strategies and management approaches form the basis for the guidelines. Because data are incomplete, however, certain aspects of these guidelines represent the current consensus judgment of the consulted experts. Recommend- ations in this document should be considered a source of guidance to health practitioners. The CDC staff members and outside experts listed below served as authors of this document. Sevgi O. Aral, Ph.D. Centers for Disease Control Robert C. Brunham, M.D. University of Manitoba Willard Cates, Jr., M.D., M.P.H. Centers for Disease Control David A. Eschenbach, M.D. University of Washington School of Medicine Michelle Y. Farmer, M.D. Baltimore City Health Department Sebastian Faro, M.D., Ph.D. Baylor College of Medicine James L. Gale, M.D. University of Washington School of Public Health and Community Medicine David A. Grimes, M.D. University of Southern California School of Medicine King K. Holmes, M.D., Ph.D. University of Washington School of Medicine Polly A. Marchbanks, Ph.D. Centers for Disease Control Zell A. McGee, M.D. University of Utah Medical Center Sam A. Nixon, M.D. The University of Texas Health Science Center at Houston Paul J. Wiesner, M.D. Dekalb County Board of Health James N. Pasley, Ph.D. University of Arkansas for Medical Sciences Dorothy S. Patton, Ph.D. University of Washington School of Medicine Herbert B. Peterson, M.D. Centers for Disease Control Peter A. Rice, M.D. Boston University School of Medicine Robert T. Rolfs, Jr., M.D. Centers for Disease Control Dennis Sayers Ohio Department of Health Julius Schacter, Ph.D. University of California San Francisco (UCSF) School of Medicine David Soper, M.D. Medical College of Virginia Richard L. Sweet, M.D. UCSF School of Medicine
Judith N. Wasserheit, M.D., M.P.H. National Institutes of Health Lars Westrom, M.D., M.P.H. University of Lund (Sweden) Steven Witkin, Ph.D. Cornell University Medical Center
Each year approximately 1 million women in the United States experience an episode of symptomatic pelvic inflammatory disease (PID)
Many women with PID have minimal symptoms, and some are believed to experience no symptoms ("silent PID"). Concern about asymptomatic PID stems from high rates of PID sequelae such as tubal infertility among women with serologic evidence of previous sexually transmitted infections but no history of overt illness. Whether such patients were truly asymptomatic or unrecognized only because of subtle or atypical clinical signs is uncertain. In either case, the best strategies for preventing PID are: a) prevention of lower-genital-tract infection with Chlamydia trachomatis and Neisseria gonorrhoeae among both men and women, b) when this fails, early detection of lower-tract infection followed by prompt and effective treatment. Implementing these two strategies requires the establishment and maintenance of effective sexually transmitted disease (STD) control programs nationally and locally. Along with appropriate medical management of illness, essential elements of such programs include: a) educating individuals to adopt healthy behaviors, b) training clinicians to counsel patients about risky behavior, c) screening persons at risk of STD, and d) involving male partners in prevention and management plans. II. MICROBIAL ETIOLOGY AND PATHOGENESIS
PID, and most cases of PID are associated with more than one organism (7-9). C. trachomatis, N. gonorrhoeae, and a wide variety of anaerobic and aerobic bacteria are recognized as playing an etiologic role for PID in the United States. Mycoplasmas have also been recovered from the genital tract, but their role in PID is less clear (10). The proportion of women with PID who are infected with C. trachomatis or N. gonorrhoeae varies widely, probably because of variations among the populations studied, differences in the time intervals of the investigations, variations in the severity of infection, and differing methods of microbial investigation. In the United States, C. trachomatis has been recovered from the cervix of 5%-39% of women diagnosed as having PID and from the fallopian tubes among zero to 10% of patients with PID (4). Serologic evidence of C. trachomatis infection has been found among 20%-40% of women with a history of PID. N. gonorrhoeae has a particularly wide range of recovery rates among women with PID, with isolation rates from the cervix ranging from 27% to 80% and from the fallopian tubes ranging from 13% to 18% (4). However, sampling of microorganisms from the fallopian tube has been difficult. In addition to C. trachomatis and N. gonorrhoeae, a wide variety of anaerobic and aerobic (facultative) bacteria have been isolated from the upper-genital tracts of 25%-50% of women with acute PID. The most common anaerobic bacteria found are Bacteroides, Peptostrep- tococcus, and Peptococcus species, whereas the most common facultative bacteria are Gardnerella vaginalis, Streptococcus species, Escherichia coil and Haemophilus influenzae. The syndrome bacterial vaginosis (BV) has also been suggested as an antecedent to lower-genital-tract infection that leads to polymicrobial acute PID (8); the organisms involved in BV are similar to the nongonococcal, nonchlamydial bacteria frequently isolated from the upper-genital-tract of women with acute PID. B. Pathogenesis PID is believed to result from direct canalicular spread of organisms from the endocervix to the endometrial and fallopian tube mucosa (9). Both N. gonorrhoeae and C. trachomatis commonly cause endocervicitis. Between 10% and 40% of women not treated for gonococcal or chlamydial cervicitis apparently develop clinical symptoms of acute PID (11,12). Even higher percentages of ascending infection are detected if endometrial biopsies are used to diagnose subclinical endometritis. Noncanalicular spread of cervical infections has also been observed, possibly extending via parametrial lymphatics (9). At least four factors could contribute to the ascent of these bacteria and/or be associated with the pathogenesis of upper-genital- tract infection. First, uterine instrumentation (e.g., the insertion of an intrauterine device {IUD}) facilitates upward spread of vaginal and cervical bacteria. Second, the hormonal changes during menses, as well as menstruation itself, leads to cervical alterations that may result in loss of a mechanical barrier preventing ascent (13). Also, the bacteriostatic effect of cervical mucus is lowest at the onset of menses. Third, retrograde menstruation may favor ascent to the tubes and peritoneum. Finally, individual organisms may have potential virulence factors associated with the pathogenesis of acute chlamydial and gonococcal PID (9,14). III. MAGNITUDE OF THE PROBLEM OF PID
afflicted with symptomatic PID. From 1979 through 1988, an annual mean of 276,100 women were hospitalized for PID, with approximately 182,000 of these hospitalizations attributed to acute PID and the remainder to chronic PID (1). During the same period, women with acute PID made an annual mean of approximately 1.2 million visits to private physicians' offices; approximately 420,000 of these representing a woman's initial visit for PID. However, comparable data on the number of women seen in public clinics, emergency rooms, and hospital outpatient departments in this 10-year period are not available. Sexually active women in younger age groups have higher rates of hospitalization for acute PID but lower rates of hospitalization for chronic PID. Data regarding office visits for PID show the age distribution to be similar to that of hospitalizations for acute PID. Women of other than white race have higher average annual hospitaliza- tion rates than white women for both acute and chronic PID. Similarly, rates of office visits for PID are slightly higher for women of other than white race (1). For acute PID, hospitalization rates are higher for women who are single, separated, or divorced than for women who are married or widowed. For both acute and chronic PID, average annual hospitalization rates are highest in the South and lowest in the Northeast, with intermediate rates in the Midwest and West. Overall, hospitalization rates for acute PID declined in the 1980s, although office-visit rates appear to have remained unchanged (1). Hospitalization rates decreased 36% from 1979 to 1988, from 3.5 to 2.2 hospitalizations per 1,000 women (Figure_1). Although hospitalization rates decreased for all age groups, a relatively smaller decrease among 15- to 19-year olds (10%) compared with a 40% decrease for the 20- to 24-year age group resulted in the younger age group's having the highest hospitalization rate in 1987-1988, even without adjusting for the proportion of sexually active women. Finally, hospitalization rates for women of both racial groups decreased similarly over the decade, and hospitalization rates declined in all four geographic regions. B. Financial Impact PID and its consequences represent a substantial economic burden. For 1990, the total cost of PID has been estimated to exceed $4.2 billion, with approximately $2.7 billion for direct expenditures for medical services (2). Annually, women make an estimated 2.5 million outpatient visits to both public and private providers for treatment for PID. More than 275,000 women are hospitalized annually for PID, with over 100,000 surgical procedures performed. In addition to these sizeable direct medical costs, indirect costs are estimated to have exceeded $1.5 billion in 1990 because of lost wages and the lost value of household management. Overall, private insurance covers approximately 41% of PID direct costs, public sources (e.g., Medicaid) 30%, health maintenance organizations/preferred provider organizations (HMO/PPO) 18%, and self payment 11% (2). Among women ages 19 years and younger, however, public sources (36%), followed by private insurance (32%), covers most PID-associated direct costs. Projections of PID costs show that by the year 2000, the total annual cost of PID will exceed $9 billion, assuming no change in PID incidence, and a constant rate of medical inflation (8.1%) (2) (Figure_2). If the incidence of PID increases by 1%/year over the 10-year period, total costs (direct and indirect) are projected to exceed $10 billion in the year 2000 (Figure_2). With a 1%/year decrease in the rate, total costs of $8.4 billion are projected. If a more substantial decrease (10%) in the rate of PID/year occurred, total costs could decline to approximately $3.2 billion. IV. RISK ASSESSMENT Identifying factors associated with increased risk of PID can help in both prevention and management of PID. Men and women without genital infections who are not engaged in high-risk sex practices can be encouraged to maintain healthy behaviors; those without infection who do acknowledge high-risk behaviors can be targeted for more intensive education and counseling. For women suspected of having PID, information about risk indicators should help clinicians in diagnosing PID when more definitive diagnostic capability, such as laparoscopy, is not readily available. However, diagnosis should not be based solely on knowledge about a suspected risk factor. Many women perceived to be at increased risk because of the presence of a risk indicator will not have PID, and many women who do not fit a typical risk profile will have PID.
residence have been correlated with risk of PID (15) (Table_1). Age is inversely related to PID rates and directly correlated with PID sequelae, (e.g., tubal damage and infertility) (4). Sexually experienced teenagers are three times more likely to be diagnosed as having PID than are 25- to 29-year-old women (16). Both biological and behavioral characteristics of adolescents may account for these differences (17). Low levels of education, unemployment, and low income as measures of socioeconomic status have been associated with increased risk of PID (15). However, the extent of the relationship between lower- genital-tract infection and socioeconomic status is not known. Data on marital status indicate that women who never married and women who are divorced or separated are at increased risk of PID (15). Finally, urban residence is often suggested to be associated with increased risk of PID, but no studies have compared PID rates among urban and rural populations. B. Individual Behavior and Practices
Provider Behavior and Practices Clinicians can help reduce the risk for PID and its sequelae. Timely diagnosis and appropriate treatment of lower-genital-tract chlamydial and gonococcal infection among both men and women can reduce the risk of adverse consequences among infected individuals and can reduce the risk of further transmission to others. Also, practitioners can influence men's and women's risk of infection by providing effective counseling about their sexual behavior, health- care-seeking behavior, and contraceptive practice, and by convincing them to comply with management instructions. Finally, by ensuring timely and effective treatment of patients' sex partners, practitioners can reduce risk of reinfection. Moreover, because the partners' infections may be asymptomatic, interviewing and treating these persons will help reduce further transmission of infection in the community and may facilitate identifying other infected persons. V. PREVENTION Preventing PID and its sequelae can take place on three levels -- primary, secondary, and tertiary prevention (37). Primary prevention involves avoiding acquisition of sexually transmitted infections. Secondary prevention involves preventing a lower-genital-tract infection from ascending to the upper-genital-tract. Tertiary prevention involves preventing upper-genital-tract infection from leading to tubal dysfunction/obstruction and functional or structural damage to other abdominal/pelvic organs. At each of these levels of prevention, communities, individuals, and health-care providers can play a role (Table_2 and Table_3).
succeed. Community-based approaches to STD/PID prevention should be aimed at providing a) information, b) motivation, and c) skills to consumers and providers. In addition, communities have a respon- sibility to provide supportive services for prevention programs. A vital element of any community strategy for prevention of PID is a community STD-control program to prevent lower-genital-tract chlamydial and gonococcal infection. Such programs are important in reducing both symptomatic and asymptomatic PID. The components of an STD-control program have been described in a variety of recent publications (38). Several of these components (counseling, disease detection, and treatment) are covered in the patient-management sections of this document. Other community activities specific to preventing PID are discussed below.
Collaboration between relevant organizations should be developed, and joint ventures between public and private entities should be encouraged at the community level. Community-based organizations are qualified to assist in prevention activities, because many of them represent or provide services to at-risk groups who are more difficult to reach through traditional STD/PID prevention channels. Relevant organizations may operate through a variety of settings, including schools, workplaces, health-care systems, religious settings, and clubs. Appropriate clinical services. High-quality clinical care that is available and accessible to persons with STD should be developed and maintained. The final common pathway for preventing both lower- and upper-genital-tract infections is the provision of effective clinical STD services, including laboratory support (42). Communities have the ultimate responsibility for ensuring access to medical care. In the United States, public STD clinics have encountered a dual problem -- an increasing demand for clinical attention to the wider STD spectrum (e.g., counseling and testing for HIV, Pap-smear screening, and drug and family- planning counseling) and decreasing resources (e.g., experienced personnel, adequate space, new funds for expanded screening) with which to address the demand. Other clinical settings, (e.g., family-planning clinics and emergency rooms in public hospitals) are also facing this dilemma. Therefore, fewer patients with STD are being evaluated and treated promptly. For example, among STD clinics in areas with the highest syphilis rates, during 1989 nearly 75% reported longer patient-waiting times and a greater number of STD patients turned away than in 1988 (CDC, unpublished data). Partner notification. Partner notification implies a public health process that informs persons directly exposed to an STD of their status so they may be evaluated and treated. Sex partners of patients with chlamydial and gonorrheal infection need to be identified for appropriate treatment. Male partners of women with PID have infection rates ranging up to 53% for chlamydial infections and 41% for gonorrhea (43; CDC, unpublished data) although the role of partner notification in directly preventing PID remains unclear. Partners can be notified in one of two ways -- patient referral or provider referral.
Training of health-care providers. Training in controlling STD problems is essential if the medical community is to support control programs. However, in the United States, medical schools have been slow to respond to the increasing magnitude of the STD problem. In 1985, only one in five medical schools provided even one-half its students with STD clinical training (CDC, unpublished data). Physicians interested in STD will need to complement their traditional diagnostic and therapeutic skills with training in behavioral science. Skills such as those required to obtain an appropriate and complete sex history, including details about sex practices and partners, must be taught. To facilitate these efforts, STD prevention and training centers have been established throughout the United States as collaborative efforts among selected local health departments, selected local academic institutions, and CDC (46). Detecting asymptomatic STD. Routine screening for chlamydial and gonococcal infection is indicated for selected groups and settings (47-49). Early detection of lower-genital-tract infection is crucial in preventing PID. Many infected women have no recognized symptoms (and often neither do their sex partners). To make the most effective use of resources, targeted screening for chlamydial infection and gonorrhea is recommended in a) adolescent-health and family-planning clinics serving high percentages of young persons, b) high-prevalence groups such as commercial sex workers and illicit drug users, and c) facilities in which high levels of STD might be expected (e.g., jails, emergency rooms). Recommended Strategies for Individuals Because of the importance of personal health behaviors, individuals must assume an active role in self-protection (Table_2). Most of these recommendations are based only on expert opinions since few prevention strategies for individuals have been appropriately evaluated.
Recommended Strategies for Health-Care Providers Providers should play a leading role in preventing PID and its sequelae (Table_3). Therefore, clinicians must assume a greater responsibility for such primary prevention activities as counseling, patient education, and community awareness, in addition to their traditional role of diagnosing illness and treating patients (49). Providing effective preventive care to patients at risk for STD involves the following five recommendations.
Recommended Strategies for Adolescents Adolescents are highly vulnerable to acquiring STD and their complications because of biological and behavioral factors. Therefore, within the context of broader community and health-provider prevention efforts, special attention should be given to adolescents. Such a focus should include the activities described below.
DIAGNOSIS Clinical diagnosis of PID is difficult because of the wide variation in symptoms and signs among women with this condition. Many women with PID may exhibit subtle, vague, or mild symptoms that are not readily recognized as PID. This situation interferes with timely diagnosis, inhibits effective treatment, and contributes to inflam- matory sequelae in the upper-reproductive tract. Laparoscopy can be used to obtain a more accurate diagnosis of salpingitis and a more complete bacteriologic diagnosis. However, this diagnostic tool is often neither readily available for acute cases nor easily justified when symptoms and signs are mild and/or vague. Moreover, laparoscopy will not detect endometritis and may not detect subtle inflammation of fallopian tubes. Consequently, the diagnosis of PID is often based on clinical findings supplemented with results of cultures or non-culture tests of samples obtained from the endocervix. The clinical diagnosis of PID is imprecise. In published studies, when compared with laparoscopy as the standard, a clinical diagnosis of symptomatic PID has a predictive value positive of approximately two-thirds. No single historical, physical, or laboratory finding is both sensitive and specific for the diagnosis of PID (i.e., can be used both to detect all cases of PID and to exclude all women without PID) (54). Combinations of diagnostic findings, which improve either sensitivity (detect more women who have PID) or specificity (exclude more women who do not have PID), have done so only at the expense of the other (i.e., requiring two or more findings will exclude more women without PID, but will also reduce the number of patients with PID who are detected). Current evidence indicates that many episodes of PID are unrecog- nized. Although some women may have truly asymptomatic ("silent") PID, others go undiagnosed because they or their health-care providers fail to recognize the implications of mild or nonspecific symptoms and/or signs. Because of the potential for damage to the reproductive health of women by even these apparently mild cases of PID, a "low threshold for diagnosis" of PID is recommended. The following recommendations for diagnosing PID are intended to help clinicians both recognize when PID should be suspected and gain additional information to increase their diagnostic certainty. Treatment for PID should be instituted on the basis of these minimum clinical criteria for pelvic inflammation in the absence of competing diagnoses (e.g., positive pregnancy test, acute appendicitis). Minimum Criteria for Clinical Diagnosis of PID
Among women with severe clinical signs, more elaborate diagnostic evaluation is warranted because incorrect diagnosis and management may cause unnecessary morbidity. Thus, additional criteria should be used to increase the specificity of diagnosis. Routine criteria are those that are simple to assess; elaborate criteria are more definitive but are more expensive and often invasive. Additional Criteria Useful in Diagnosing PID Routine Elaborate
bacteriologic diagnosis is helpful. It provides diagnostic confir- mation (thereby improving management and reinforcing the need to treat sex partners) and serves as baseline for test-of-cure cultures. Tests Recommended for All Suspected Cases of PID
OTHER IMPORTANT DIAGNOSTIC CONSIDERATIONS The diagnostic approach outlined above reflects a growing concern that PID is often not diagnosed, especially among women with mild or atypical clinical signs. Although correcting this situation is a high public health priority, three qualifications regarding this more sensitive diagnostic approach must be noted. First, the use of highly sensitive PID diagnostic criteria means that many women who do not have PID will be misdiagnosed and treated for PID (low specificity). Patients and their sex partners often have strong emotional reactions when faced with the implications of a diagnosis of STD. The health-care provider must, therefore, inform a patient of a diagnosis of PID carefully. Both the uncertainty of the diagnosis and the value of empiric treatment must be explained clearly. Second, careful follow-up is necessary. If no clinical improvement has occurred at 48-72 hours, alternate diagnoses (e.g., appendicitis, endometriosis, ruptured ovarian cyst, or adnexal torsion) should be reconsidered. Use of alternate or additional antimicrobial therapy should also be considered. Third, use of even these minimum clinical criteria may exclude some women with PID. Clinicians should not withhold therapy from a woman in whom they suspect PID because of failure to meet these criteria. VII. TREATMENT
management. Practitioners should explain to women the nature of their disease and should encourage them to comply with therapy and prevention recommendations. Specifically, practitioners should:
rigorously delivered by providers, patients are likely to comply (56). Reinforcement of these messages can be achieved by providing written information. Information on written materials for patient distribution can be obtained from CDC or local and state health departments. ** B. Management of Sex Partners Treatment for sex partners of women with PID is imperative. The management of women with PID should be considered inadequate unless their sex partners have been appropriately evaluated and treated. Failure to manage her sex partner(s) effectively places a woman at risk for recurring infection and related complications. Moreover, untreated sex partners often unknowingly transmit STD in a community because of asymptomatic infection. In clinical settings in which only women are seen, special arrangements should be made to provide care for male sex partners of women with PID. When this is not feasible, clinicians should ensure that sex partners are referred for appropriate evaluation and treatment. After evaluation, sex partners should be empirically treated with regimens effective against C. trachomatis and N. gonorrhoeae infections (48). C. Hospitalization The efficacy of outpatient management for preventing late sequelae remains uncertain. A single intramuscular (IM) injection of cefoxitin or ceftriaxone, even in conjunction with oral doxycycline for 10-14 days, will provide less complete antimicrobial coverage for a shorter duration than regimens recommended for inpatients. Theoretically, outpatient management could, therefore, reduce the likelihood of successful eradication of upper-genital-tract pathogens and potentially increase the likelihood of late sequelae. Currently, no data are available to adequately assess the risks, benefits, and costs of inpatient versus outpatient treatment for PID. As for all serious intra-abdominal infections, hospitalization should be considered whenever possible, and is particularly recommended in the following situations:
so that treatment with parenteral antibiotics can be initiated. D. Treatment Regimens Although several antimicrobial regimens have been proven highly effective in achieving clinical cure, no single therapeutic regimen of choice exists for persons with PID (57), unlike treatment for many specific sexually transmitted organisms (48). PID is a complex syndrome that encompasses a broad spectrum of inflammatory diseases (e.g., endometritis, salpingitis, and tubo-ovarian abscess) that may be caused by a variety of organisms. Guidelines for the treatment of patients with PID, therefore, have been designed to provide flexibility in therapeutic choices. PID therapy regimens are designed to provide broad-spectrum coverage of likely etiologic pathogens. In addition to considering microbial etiology, selection criteria for a treatment regimen should also include institutional availability, cost-control efforts, patient acceptance, and regional differences in antimicrobial susceptibility. The treatment regimens that follow are recommendations, and the specific antibiotics named are examples. Treatments used for persons with PID will continue to be broad spectrum until more definitive studies are performed. Any regimen used, however, should cover C. trachomatis, N. gonorrhoeae, anaerobes, gram-negative rods, and streptococci.
Rationale Clinicians have extensive experience with both the cefoxitin/ doxycycline and clindamycin/aminoglycoside combinations. Each of these regimens provides broad coverage against polymicrobial infection and has been shown in numerous studies to be highly effective in achieving clinical cures. However, data are lacking on the efficacy of these regimens, as well as other regimens, in preventing late sequelae. Cefotetan has properties similar to those of cefoxitin and requires less frequent dosing. Clinical data are limited on other third- generation cephalosporins (ceftizoxime, cefotaxime, ceftriaxone), to replace cefoxitin or cefotetan, although many authorities believe they are effective. Doxycycline administered orally has bioavailability similar to that of the IV formulation and may be given if normal gastrointestinal function is present. Experimental studies suggest that aminoglycosides may not be optimal treatment for patients who have gram-negative organisms within abscesses, but clinical studies suggest that they are highly effective in treating persons for abscesses when administered in combination with clindamycin. Short courses of aminoglycosides are given to healthy young women when serum-level monitoring is usually not required. 2. Outpatient Management Recommended regimen Cefoxitin 2 g IM plus probenecid, 1 g orally, concurrently or ceftriaxone 250 mg IM or equivalent cephalosporin plus Doxycycline 100 mg orally 2 times a day for 10-14 days or Tetracycline 500 mg orally 4 times a day for 10-14 days. Alternative Regimen for Patients Who Do Not Tolerate Doxycycline/tetracycline **** Substitute erythromycin 500 mg orally 4 times a day for 10-14 days. **** Rationale These empiric regimens provide broad-spectrum coverage against the common etiologic agents of PID. Notably, these regimens were particularly designed to treat persons with chlamydial and gonococcal infections; few data are available on the efficacy of these regimens for treating persons with PID, particularly nonchlamydial/nongonococcal PID. Parenteral B-lactam antibiotics are recommended in all cases. The cephalosporins are effective in treating persons with gram- negative organisms, including enteric rods, anaerobic organisms, and gonococci. Although decreased susceptibility of gonococci to cefoxitin has recently been noted, clinically evident treatment failure has not been a problem. Patients who do not respond to therapy within 72 hours should be hospitalized for parenteral therapy. Doxycycline provides definitive therapy for chlamydial infections. Patients treated on an outpatient basis need to be monitored closely and reevaluated in 72 hours. E. Management of HIV-Infected Women Although the precise etiologic relation between HIV infection and the risk of PID is uncharacterized -- since HIV infection is sexually transmitted and PID is often caused by sexually transmitted pathogens -- these two conditions often coexist (58.59). The management of coexistent HIV infection and PID is becoming an increasingly important concern. Differences in the clinical manifestations of PID among HIV-infected women have not been clearly described. However, PID among women immunocompromised for any reason may be more clinically severe and more refractory to medical management than PID among women with normal host defenses. It is reasonable to expect, therefore, that those HIV-infected women who are immunocompromised may be at increased risk for a complicated clinical course. In one study, HIV-infected women with PID were less likely than HIV-negative women with PID to have an elevated white-blood-cell count, but were more likely to have tubo-ovarian abscesses and to require operative intervention (60). HIV-infected women who develop PID should be followed closely with early hospitalization and IV therapy with a recommended antibiotic regimen, if possible. VIII. SURVEILLANCE Currently, data for surveillance of PID are derived primarily from surveys of reproductive-age women, hospital discharges, or visits to physicians. Although these surveys offer important national estimates of the number of PID diagnoses, information at the local level is necessary to determine the magnitude and nature of PID in a particular community, to plan prevention activities based on local needs, and to evaluate the success of control strategies. At all levels, PID surveillance is affected by four main constraints:
quickly resolved, universal communicable disease reporting is unlikely to be a suitable model for PID surveillance. The objectives of PID surveillance at the national, state, and local levels are to provide quantitative estimates of disease occurrence, to determine secular trends, to target intervention resources, and to evaluate control efforts (61). RECOMMENDATIONS
Disease reporting can be ongoing or systematically episodic (e.g., reporting could occur for 6 weeks of every year). Ideally, to determine the disease burden of PID in a community, sentinel practitioners and sites should be selected in a way that permits the total number of PID cases in the community to be estimated. However, this type of estimation requires a detailed sampling scheme that is difficult to apply in most community settings. An alternative to a community-based surveillance system would be to use as a sentinel site a health-maintenance organization or other health-care provider that serves a well-characterized population. In other settings, in which population-based estimates are not possible, sentinel sites may still be useful for monitoring trends.
** Information Services, Center for Prevention Services, Centers for Disease Control, E06, Atlanta, Georgia 30333. Telephone (404) 639-1819. *** Other cephalosporins such as ceftizoxime, cefotaxime, and ceftriaxone, which provide adequate gonococcal, other gram- negative aerobic, and anaerobic coverage, may be utilized in appropriate doses. **** No data available on this regimen. ***** Information Services, Center for Prevention Services, Centers for Disease Control (E06), Atlanta, Georgia 30333. Telephone (404) 639-1819. References
Figure_1 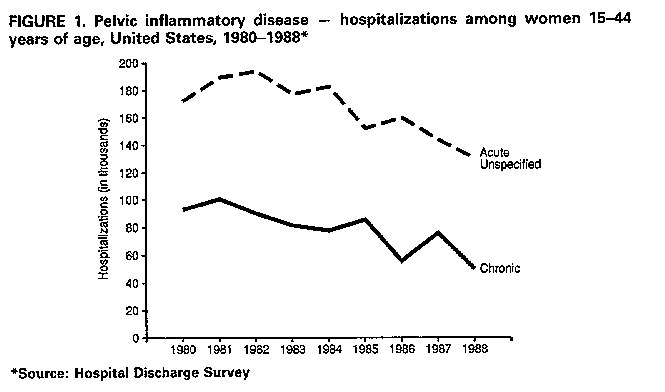 Return to top. Figure_2 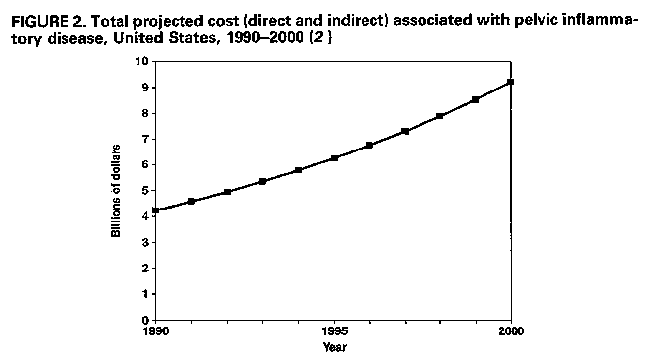 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Health outcomes affected (15)
===================================================================================
Progression of disease
------------------------------------------
Acquisition Development Development of
Risk Variable of STD of PID PID sequelae
---------------------------------------------------------------------------------
Demographic and social indicators
Age + + -
Socioeconomic status + + .
Marital status + + .
Residence, rural or urban + . .
Individual behavior and practices
Sexual behavior
Number of partners + . .
Age at first sexual intercourse + . .
Frequency of sexual intercourse + . .
Rate of acquiring new partners + . .
Contraceptive practice
Barrier - - -
Hormonal + - .
Intrauterine device . + +
Health-care behavior
Evaluation of symptoms + + +
Compliance with treatment
instructions + + +
Partner notification + + +
Others
Douching . + .
Smoking + + .
Substance abuse + . .
Menstrual cycle + + .
---------------------------------------------------------------------------------
Key: (+) increased risk; (-) decreased risk; (.) no association reported.
===================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Recommendations for individuals to prevent STD/PID (37)
=================================================================================================
Quality of
General evidence supporting
preventive effectiveness of
measures Specific Recommendations intervention *
-----------------------------------------------------------------------------------------------
Maintain healthy Postpone intitiation of sexual intercourse until III
sexual behavior at least 2-3 years following menarche
Limit number of sex partners II
Avoid "casual" sex and sex with high-risk partners III
Question potential sex partners about STD and III
inspect their genitals for lesions or discharge
Avoid sex with infected persons III
Abstain from sex if STD symptoms appear III
Use barrier Use condoms, diaphragms, and/or vaginal II
methods spermicides for protection against STD,
even if contraception is not needed.
Use condoms consistently and correctly II
throughout all sex
Adopt healthy Seek medical evaluation promptly after having III
medical-care- unprotected sex (intercourse without a condom)
seeking behavior with someone who is suspected of having an STD
Seek medical care immediately when III
genital lesions or discharge appear
Seek routine check-ups for STD if in non-mutually III
monogamous relationship(s), even if symptoms
are not present
Comply with Take all medications as directed, regardless I
management of symptoms
instructions
Return for follow-up evaluation as instructed III
Abstain from sex until symptoms disappear III
and appropriate treatment is completed
Ensure examination When diagnosed as having an STD, notify III
of sex partners all sex partners in need of medical assessment.
If preferred, assist health providers in identifying III
sex partners
-----------------------------------------------------------------------------------------------
Key: I: Evidence obtained from at least one properly randomized controlled trial; II: Evidence
obtained from well-designed cohort or case-control analytic studies; III: Opinions of respected
authorities based on clinical experience, descriptive studies, or reports of expert committees.
* Source: U.S. Preventive Services Task Force.
=================================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Recommendations for health providers to prevent STD/PID (37)
============================================================================================
General preventive measures Specific recommendations
------------------------------------------------------------------------------------------
Maintain up-to-date knowledge Develop an accurate base of information on the
about the prevention and diagnosis, treatment, and prevention of STD/PID
management of STD/PID
Complete continuing education courses periodically
to update knowledge on STD/PID prevention and
management
Provide effective patient Educate patients about STD/PID and their
education and counseling potential complications
Encourage individuals to maintain healthy sexual
behavior, use barrier methods, and adopt healthy
medical-care-seeking behavior
Provide appropriate preventive Screen patients for chlamydial and gonococcal
medicine services infection routinely when indicated
Provide epidemiologic treatment for STD/PID
when appropriate
Provide appropriate medical Diagnose STD/PID promptly
management for illness
Treat STD/PID promptly and with effective antibiotics
Encourage patients to comply with management
instructions
Ensure examination Encourage infected patients to refer all sex
of sex partners partners in need of medical assessment
Evaluate and treat sex partners appropriately
Report all STD to appropriate
health authorities
------------------------------------------------------------------------------------------
============================================================================================
Return to top. Disclaimer All MMWR HTML documents published before January 1993 are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 08/05/98 |
|||||||||
This page last reviewed 5/2/01
|