 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. International Notes Bolivian Hemorrhagic Fever -- El Beni Department, Bolivia, 1994In July 1994, an outbreak of Bolivian hemorrhagic fever (BHF), which is caused by Machupo virus, began in northeastern Bolivia. This report describes the investigation and features of this outbreak, the search for additional cases of BHF in El Beni, Bolivia, and results of rodent investigations. Initial Investigation The outbreak initially occurred among members of an extended family residing in Magdalena (1994 population: approximately 5300) located in the north central Province of Itenez, El Beni Department Figure_1. From July 4 through August 12, 1994, seven family members (aged 10 months-50 years) developed an illness characterized by fever, hypotension, subconjunctival and gingival bleeding, epistaxis, petechiae, tremor, and dysarthria. Six of these persons died; the person who had the index case survived. Laboratory studies performed on serum and tissue specimens from decedents confirmed the diagnosis of BHF by isolation of Machupo virus and detection of viral antigen in all five patients for whom specimens were available; the survivor developed enzyme-linked immunosorbent assay (ELISA) immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies to Machupo virus. Search for Other Cases Following identification of the familial cluster, three additional persons in Bolivia with suspected BHF were reported to the National Secretary of Health through provincial health departments. On August 18, a broken test tube in a centrifuge exposed a 37-year-old laboratory technician in Santa Cruz to aerosolized blood from one of the family members who died. On August 29, the technician developed an acute febrile illness with lower back pain, arthralgias, and mild conjunctivitis. On August 30, intravenous therapy with the antiviral compound ribavirin was initiated for a presumptive diagnosis of BHF. She had no hemorrhagic manifestations and recovered from her illness. Machupo antigen detection and virus isolation studies on serum obtained before initiation of ribavirin treatment were negative, as were IgG and IgM antibody ELISAs on serum specimens collected 3 months after onset. On August 28, a 41-year-old man residing in Magdalena (with no known link to any infected persons) developed an illness that included fever, chills, and hip pain. On September 2, he was transferred to a hospital in Cochabamba, Bolivia, and died on September 5 following a fulminant hemorrhagic clinical course. Machupo virus was isolated and viral antigen was detected in the patient's serum. On September 3, a 52-year-old agricultural worker from Poponas, El Beni Department, developed a febrile hemorrhagic illness; on September 11, he was admitted to a hospital in Trinidad, El Beni Department. On September 13, intravenous ribavirin therapy was initiated for a presumptive diagnosis of BHF, and the patient recovered. The diagnosis of BHF was confirmed by detection of viral antigen and virus isolation from the patient's serum. Family members of these three persons with presumptive or confirmed BHF cases and health-care workers in contact with these persons were monitored for febrile illness. However, illness was not noted in these patient contacts. Rodent Investigation During August and September 1994, rodent trapping was conducted in areas of potential exposure for the affected family. During 1811 trap-nights, * 84 rodents were captured, including nine Calomys callosus. Testing for antibodies to Machupo virus was negative for each of the 84 rodents. Virus isolation studies on captured rodents are pending. Reported by: M Villagra, MD, National Hemorrhagic Fever Program, National Secretary of Health, Ministry of Human Development; L Suarez, MD, Regional Health Secretary, El Beni Department; R Arce, MD, Magdalena Hospital, Magdalena, Province of Itenez, El Beni Department, Bolivia. MG Moreira, MD, Pan American Health Organization, La Paz, Bolivia. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: BHF is a viral hemorrhagic fever known to be endemic only in Bolivia; first described in 1959, it caused outbreaks in small communities in eastern Bolivia throughout the 1960s (1). The etiologic agent, Machupo virus, is a member of the family Arenaviridae and is maintained in the rodent C. callosus, the natural reservoir (2). As with other arenaviruses, infection of the rodent host results in a persistent asymptomatic infection with shedding of virus in urine. Human infections are believed to occur following exposure to the virus in aerosolized rodent urine. A nosocomial outbreak of BHF in Cochabamba in 1971 suggested that person-to-person transmission also may occur by airborne or parenteral routes (3). Following an incubation period of 1-2 weeks, patients infected with Machupo virus may develop an influenza-like illness with fever, malaise, and fatigue followed by the onset of headache, dizziness, myalgias, and severe lower back pain. Prostration, abdominal pain, anorexia, tremors, and hemodynamic instability may be followed by hemorrhagic manifestations, including bleeding from the oral and nasal mucosa and the gastrointestinal, genitourinary, and bronchopulmonary tracts (4). BHF can be diagnosed by virus isolation from acute serum or tissue specimens or by virus antigen detection using an ELISA. Antibodies can be detected using plaque-reduction neutralization, indirect immunofluorescence, or ELISAs. Because of the risk for laboratory-acquired infections with this highly lethal agent, tests with potentially infectious material should be performed in a biosafety level 4 laboratory (5). Treatment of BHF employs supportive measures. Although uncontrolled trials have used convalescent immune plasma from survivors of BHF, evaluation of the effectiveness of this therapy has been limited by the lack of plasmapheresis capability and availability of qualified donors. Ribavirin, a broad-spectrum antiviral agent, has been effective against human Lassa fever and several arenavirus diseases in animal models. Patients infected with Junin virus, a closely related arenavirus, also have received the drug (6), but there is no definitive evidence concerning efficacy. From 1959 through 1962, Bolivian health officials reported 470 cases of BHF with 142 deaths (case-fatality rate: 30%) (7). Until the cases described in this report, the last confirmed outbreak in Bolivia occurred in 1971 (3). The mode of transmission of BHF in the familial outbreak described in this report is unclear. Although no C. callosus were captured in the town of Magdalena, a low density of C. callosus was noted in rural areas around Magdalena where the index case had worked and traveled. Previous trapping in Bolivia has shown fluctuations in population numbers and prevalence of infection among C. callosus, but the determining factors are not known (2,8). Previous cases of BHF occurred following rodent invasion of households in towns and exposure during campestral activities, including sleeping in primitive shelters (9). Infection with Machupo virus among travelers returning to the United States has not been recognized. References
* The total number of traps set in 1 night multiplied by the total number of nights during which traps were set. Figure_1 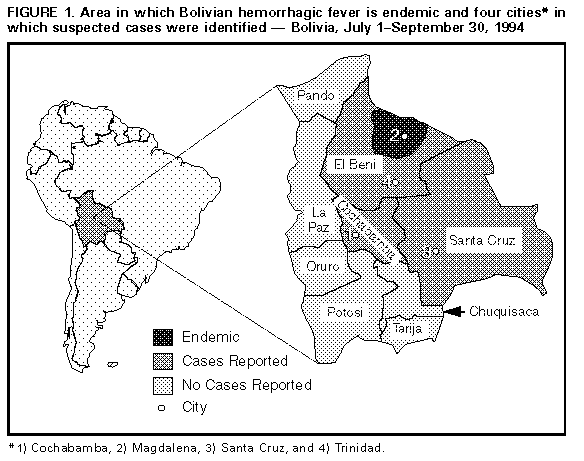 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|