 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Diphtheria Epidemic -- New Independent States of the Former Soviet Union, 1990-1994Although diphtheria was controlled for approximately 30 years after the institution of childhood vaccination with diphtheria toxoid in the late 1950s, epidemic diphtheria has reemerged in the New Independent States (NIS) of the former Soviet Union (1,2) Figure_1 and Figure_2. The epidemic began in 1990 in the Russian Federation and spread to Ukraine in 1991 and, during 1993-1994, to 12 of the 13 remaining NIS. In most affected countries, the incidence rate of reported diphtheria has increased twofold to 10-fold each year. This report summarizes data provided to the World Health Organization (WHO) about diphtheria in the NIS during 1989-1994. * Overall, reported cases of diphtheria in the NIS increased from 839 in 1989 to 47,802 in 1994 (Figure 1). In 1994, a total of 1746 persons died; case-fatality rates ranged from 2.8% (Russian Federation) to 23.0% (Lithuania and Turkmenistan). In the Russian Federation, reported diphtheria cases increased from 603 in 1989 (0.4 per 100,000 population) to 15,229 (10.3) in 1993, then increased 161% to 39,703 (26.6) in 1994; a total of 1104 (2.8%) persons died. The epidemic has progressively spread to involve all 89 administrative regions. Throughout the epidemic, approximately 70% of cases have been reported among persons aged greater than or equal to 15 years. The highest age-specific incidence rates were among persons aged 4-10 years, 15-17 years, and 40-49 years. Reported nationwide coverage with a primary series (three doses) of diphtheria toxoid among children aged 12-23 months increased from 72.6% in 1992 to 79.2% in 1993, but coverage remains low ( less than 60%) in some regions. During 1992-1993, at least 90% of children aged less than or equal to 5 years had received a primary series with diphtheria and tetanus toxoids and pertussis vaccine (DTP) or pediatric (DT) or adult (Td) formulation diphtheria and tetanus toxoids, and approximately 80% had received at least one booster. Up to 50% of infants in some areas may have received a primary series with Td rather than DTP or DT. In Ukraine, reported cases increased 27-fold, from 109 in 1990 to 2990 (5.7 per 100,000) in 1994; 111 (3.7%) persons died. In 1994, 80% of cases occurred among persons aged greater than or equal to 15 years. In Belarus, reported cases increased 97%, from 120 in 1993 to 236 (2.3) in 1994. In Moldova, reported cases increased 10-fold, from 35 in 1993 to 372 (8.5) in 1994; 19 (5.1%) persons died. In Latvia, reported cases increased 21-fold, from 12 in 1993 to 250 (9.4) in 1994 and, in Lithuania, from eight in 1993 to 39 (1.0) in 1994. In Estonia, seven cases (0.4) were reported in 1994. In Tajikistan, reported cases increased 180%, from 680 in 1993 to 1907 (31.8 per 100,000) in 1994. Most cases were reported from the southern region of Kurgan Tyube, which borders Afghanistan. For other central Asian republics, case counts for 1994 were Kazakhstan, 489 (2.8); Kyrgyzstan, 303 (6.5); Turkmenistan, 61 (1.5); and Uzbekistan, 232 (1.0). These totals represent increases of twofold to 20-fold over 1993. Approximately 50% of cases in these countries have occurred among persons aged less than or equal to 14 years. In Georgia, 294 cases (5.4 per 100,000) were reported in 1994, a 10-fold increase over 1993. Forty-two (14%) persons died, and 43% of cases occurred among persons aged greater than or equal to 15 years. In Azerbaijan, 685 cases (9.2) were reported in 1994, compared with 141 in 1993. Armenia reported no cases during 1991- 1993 but 36 (1.0) cases in 1994. Reported by: Regional Office for Europe, World Health Organization, Copenhagen. Global Program on Vaccines and Immunization, World Health Organization, Geneva. Regional Office for Eastern Europe and Central Asia, United Nations Children's Fund, New York and Geneva. Child Survival Unit, United Nations Children's Fund, New York. Childhood and Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; International Health Program Office; Child Vaccine Preventable Disease Br, Div of Epidemiology and Surveillance, National Immunization Program, CDC. Editorial NoteEditorial Note: WHO considers the rapidly expanding diphtheria epidemic in the NIS an international public health emergency. In the Russian Federation, the epidemic has intensified each successive year. In the central Asian and Transcaucasian republics, epidemic diphtheria is established in all eight countries; in some of these countries, many cases have occurred among refugees or persons displaced by internal conflict. Previous reports of diphtheria epidemics underscore the potential for further increase in the magnitude of this epidemic (3). Although the reasons for the diphtheria epidemic in the NIS are not fully understood, one important factor is the presence of a large number of susceptible children and adults -- which enabled introduction or reemergence of toxigenic strains of Corynebacterium diphtheriae. Spread of the organism may have been facilitated by crowding and population migration resulting from the dissolution of the Soviet Union. In addition, adequate control measures (particularly aggressive mass vaccination in affected areas) were not implemented during the early phase of the epidemic. Increases in the number of susceptible children in the NIS probably resulted from a combination of low vaccination coverage in many areas and inappropriate primary vaccination of substantial numbers of infants with Td, a formulation for adults containing decreased amounts of diphtheria toxoid. The existence of large numbers of susceptible adults is a new phenomenon in the vaccine era. In the prevaccine era, most persons acquired immunity to diphtheria naturally before adulthood through exposure to C. diphtheriae. Following the introduction of childhood vaccination with diphtheria toxoid, circulation of toxigenic C. diphtheriae decreased substantially. In addition, vaccine-induced immunity wanes over time unless periodic boosters are administered. Serologic studies in the NIS, Western Europe, and the United States indicate that 20%-60% of adults aged greater than or equal to 20 years are susceptible to diphtheria (4- 7). Lack of effectiveness of diphtheria toxoid is not considered to be an important contributing factor for this epidemic. Recent assessments of vaccine effectiveness in children conducted in the Russian Federation and Ukraine have documented high clinical effectiveness of diphtheria toxoid produced in the Russian Federation. A plan formulated jointly by WHO and the United Nations Children's Fund (UNICEF), in collaboration with CDC, the U.S. Agency for International Development, and the International Federation of Red Cross and Red Crescent Societies, has outlined the strategies necessary to control the diphtheria epidemic in the NIS. This plan was approved in January and February 1995 by representatives of the affected countries. Key elements of the plan are to achieve and maintain high levels of routine childhood vaccination with diphtheria toxoid (i.e., greater than or equal to 95% coverage with four doses of DTP by age 2 years in all districts and the same levels for booster doses according to national vaccination schedules) and to rapidly vaccinate greater than or equal to 90% of adolescents and adults with Td (8,9) in all areas of the NIS affected by the epidemic. Because of widespread transmission among the entire population of the NIS, attempts to control the epidemic through vaccination of targeted subgroups (e.g., adults in selected occupations and children) have not been effective. In 1994, at least 20 imported cases of diphtheria were reported in countries in Europe, including Bulgaria, Finland, Germany, Norway, and Poland. This demonstrates the potential for the diphtheria epidemic in the NIS to spread to neighboring countries in Europe, the Middle East, and Asia. Although no cases have been directly imported into the United States, CDC has received reports of two cases of diphtheria among U.S. citizens who reside in or who traveled to the NIS, and considers the epidemic to pose a risk for importation into the United States. This report underscores the importance of maintaining high levels of diphtheria immunity among the total populations of the United States and other countries, regardless of whether international travel is planned. The Advisory Committee on Immunization Practices recommends that all children receive a routine series of five doses of DTP (or DT if pertussis vaccine is contraindicated) with doses at ages 2, 4, 6, and 12-15 months and 4-6 years; Td boosters should then be administered every 10 years (10). For persons aged greater than or equal to 7 years who have not been previously vaccinated against diphtheria, the primary series consists of three doses of Td, with intervals of 1-2 months between the first two doses and 6-12 months between the second and third doses. Persons traveling to areas with diphtheria activity should have completed the primary series and should have received the most recent dose of vaccine (either primary series or booster) within the previous 10 years. Control of the epidemic in the NIS requires immediate efforts to raise levels of immunity through extensive mass vaccination of adolescents and adults. Shortages of vaccine, antitoxin, and antibiotics exist in the NIS (except the Russian Federation); these needs should immediately be addressed through coordinated efforts of international public health and donor agencies. An Interagency Immunization Coordinating Committee was convened in 1994 and is scheduled to meet again in April 1995 to coordinate donor activities in support of disease control and primary vaccination of children in the NIS. References
* Data for 1994 are provisional. Figure_1 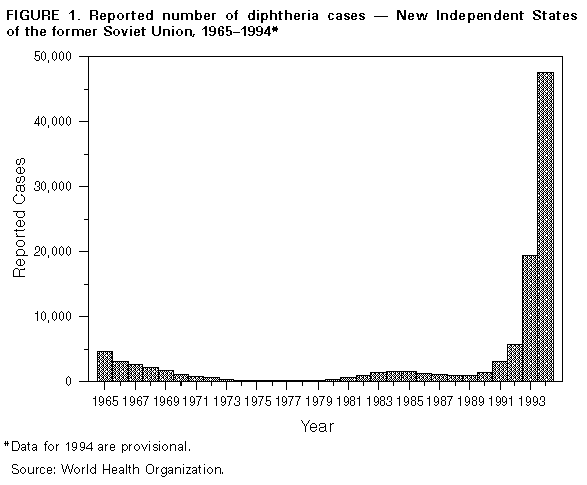 Return to top. Figure_2 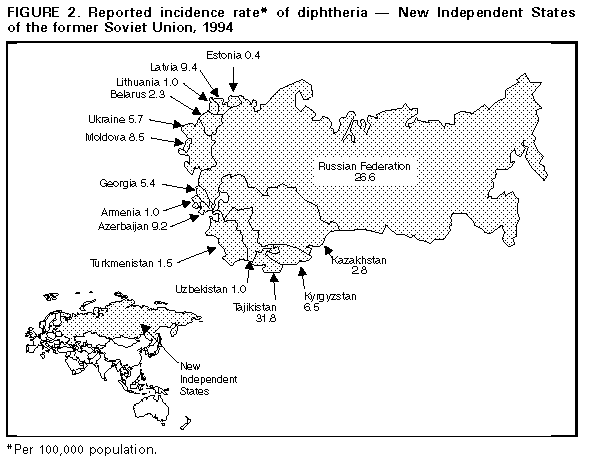 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|