 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Statewide Surveillance for Antibiotic-Resistant Bacteria -- New Jersey, 1992-1994The increasing occurrence of infection with antibiotic-resistant microorganisms and other emerging infectious diseases has required the development of flexible and timely surveillance systems for monitoring these problems (1,2). To determine the extent of antibiotic resistance in New Jersey, in 1991 the New Jersey State Department of Health (NJSDOH) initiated a hospital laboratory isolate-based surveillance system for antimicrobial-resistant bacteria. This report describes the surveillance system and summarizes findings during 1992-1994 for vancomycin-resistant enterococci (VRE) -- the most rapidly increasing antibiotic-resistant bacteria reported by New Jersey hospitals. The surveillance system includes the 95 acute-care hospitals licensed by the state of New Jersey. Organisms targeted for surveillance include gram-positive cocci resistant to vancomycin, including VRE; methicillin-resistant Staphylococcus aureus (MRSA); gram-negative rod-shaped bacteria (GNRs) resistant to imipenem; GNRs resistant to amikacin; and pneumococcal and other streptococcal isolates resistant to penicillin. Hospitals submit to NJSDOH monthly a surveillance report form, which includes the number of in-patient bloodstream isolates of these organisms and MRSA isolates from any body site. The New Jersey Administrative Code, which addresses communicable diseases, and state hospital licensure standards were modified in 1990 to require hospitals to submit these data to NJSDOH. Hospitals are contacted by the surveillance system coordinator to ensure monthly reporting; since the surveillance system was initiated, all hospitals have submitted monthly reports (3). During 1992-1994, a total of 5916 (81%) bloodstream isolates reported to this system were MRSA. Of the 1398 non-MRSA bloodstream isolates, 663 (47%) were VRE. During this period, both the number of hospitals reporting VRE blood isolates and the number of VRE isolates increased steadily: in 1992, 33 hospitals reported 99 isolates, while in 1994, 54 hospitals reported 278 isolates Figure_1. Most of the monthly reports (73%) represent only one reported isolate per hospital. In 1992, hospitals in 13 of the 21 counties reported VRE isolates, compared with 20 of 21 counties in 1994. Reported by: SM Paul, MD, L Finelli, DrPH, G Crane, MPH, KC Spitalny, MD, State Epidemiologist, New Jersey State Dept of Health. National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: The recent national emphasis on emerging infectious diseases has underscored the problem of antibiotic resistance involving a variety of nosocomial and community-acquired infections and has focused attention on the importance of microbiology laboratories as sources of surveillance information for antibiotic resistance (1,2). For example, in New Jersey, the increase in both the number of VRE blood isolates and the number of hospitals reporting VRE blood isolates from 1992 through 1994 suggests the emergence of the problem of VRE in that state. Careful monitoring of such trends in antibiotic resistance in enterococci and other organisms assists clinicians in selecting antibiotics for their patients and public health agencies in the development and implementation of prevention efforts. In New Jersey, laboratory-based surveillance for VRE and other antibiotic-resistant isolates has been developed through collaboration between the NJSDOH, hospitals, and infectious disease professionals in the state and because of modification of reporting regulations. The New Jersey system uses data that are routinely collected and collated by hospital laboratories and requires few additional resources. Because this surveillance system is isolate-based, it does not directly measure changes in the rate of infection in persons, and NJSDOH has used this system primarily for sentinel purposes to guide further investigation. For example, early detection and geographic tracking of VRE in New Jersey through this system have facilitated collaborative efforts involving public and private sector and academic organizations to evaluate risk factors for VRE, treatment options, VRE in vitro susceptibility to antimicrobial agents before clinical trials, and the effectiveness of infection-control practices (4-7). These efforts have, in turn, enabled the NJSDOH to collaborate with professional organizations (the Infectious Diseases Society of New Jersey and the New Jersey chapters of the Association for Professionals in Infection Control and Epidemiology) to develop recommendations to prevent VRE transmission and have provided a source of bacterial isolates to assist in research efforts to develop effective antimicrobial agents against VRE. References
Figure_1 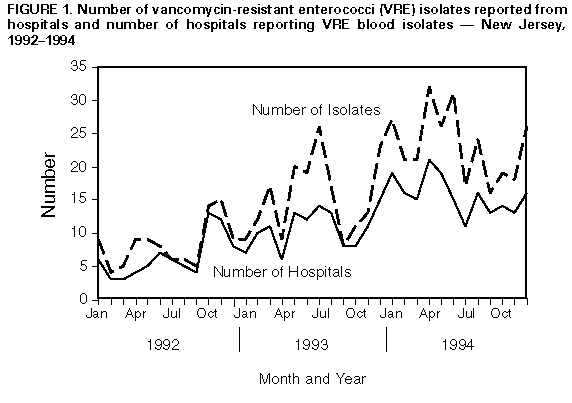 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|