 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Defining the Public Health Impact of Drug-Resistant Streptococcus pneumoniae: Report of a Working GroupSummary Streptococcus pneumoniae is a leading cause of morbidity and mortality in the United States, resulting each year in an estimated 3,000 cases of meningitis, 50,000 cases of bacteremia, 500,000 cases of pneumonia, and 7,000,000 cases of otitis media. As with most respiratory pathogens, rapid, sensitive, and specific diagnostic tests are not available; thus, early in the course of illness, diagnosis of S. pneumoniae infection is usually presumptive, and the choice of antimicrobial therapy is nearly always empiric. In the past, isolates of S. pneumoniae were uniformly susceptible to penicillin; however, penicillin-resistant and multidrug-resistant strains have begun to emerge in the United States and are widespread in some communities. The full impact of the problem is unknown because infection with drug-resistant S. pneumoniae (DRSP) is not a reportable condition for most of the United States. To develop a strategy for minimizing the impact of DRSP, in June 1994, CDC convened a working group of public health practitioners, clinical laboratorians, health-care providers, and representatives of key professional societies. This report describes the three goals developed by the working group that address surveillance, epidemiologic investigation, and prevention and control of DRSP, and the objectives for each goal. INTRODUCTION Streptococcus pneumoniae infections are among the leading causes worldwide of illness and death for young children, persons who have underlying debilitating medical conditions, and the elderly (1). Each year in the United States, pneumococcal disease is estimated to account for 3,000 cases of meningitis, 50,000 cases of bacteremia, 500,000 cases of pneumonia, and 7,000,000 cases of otitis media (2). Case-fatality rates vary by age and underlying illnesses of patients; however, case-fatality rates for some high-risk patients have been reported to be greater than 40% for bacteremia and 55% for meningitis, despite appropriate antimicrobial therapy (3). A vaccine for the 23 most common serotypes of S. pneumoniae has been available since the early 1980s. The Advisory Committee on Immunization Practices (ACIP) recommends that the vaccine be administered to persons greater than or equal to 2 years of age who have certain underlying medical conditions associated with increased risk for pneumococcal disease and its complications and to all persons greater than or equal to 65 years of age (3). However, despite its availability, the vaccine is underutilized. As of 1993, data from CDC's National Health Interview Survey indicated that only 27% of persons greater than or equal to 65 years of age had been vaccinated (4). In the past, S. pneumoniae was almost uniformly susceptible to penicillin, allowing most physicians to treat persons who had severe infections with penicillin alone without testing for resistance. Since the 1960s, however, resistance to penicillin and other antimicrobial agents has spread rapidly and was first reported in Australia in 1967, in New Guinea in 1969, in South Africa in 1977, and in many other countries throughout Africa, Asia, and Europe (5,6). In the United States, high-level resistance to penicillin has increased substantially in the last decade. Investigations of outbreaks by CDC have revealed that resistance to penicillin varies by region; in some areas of the United States, as many as 30% of pneumococcal isolates are resistant to penicillin (7,8). Also, the incidence of drug-resistant infections can change rapidly (9). A smaller yet substantial percentage of isolates is also resistant to multiple (i.e., two or more) antimicrobial drugs; some are susceptible only to vancomycin. Within communities, the proportion of pneumococcal illnesses caused by drug-resistant S. pneumoniae (DRSP) among children may be markedly different from that among adults. Therapy for invasive pneumococcal disease and for milder illnesses (e.g., otitis media) remains empiric because rapid, sensitive, and specific diagnostic tests are not available. Although the choice of antimicrobial agents for empiric therapy should be guided by the regional prevalence of DRSP, the prevalence of resistance to penicillin is unknown for most areas of the United States because DRSP infection has not been a reportable condition. Consequently, for these infections, therapy often consists of prescribing antimicrobial drugs that are either not necessary or are too broad. Inappropriate empiric or prophylactic use of antimicrobial drugs contributes to the development of DRSP. Rapid emergence of resistance to antimicrobial drugs among other bacteria also has been documented. This trend of waning susceptibility has fostered public concern, as reported in nationally syndicated magazines and recently published books (10,11). Treating patients who have resistant organisms often requires both prolonged hospitalization and the use of expensive alternative antimicrobial drugs. New drugs to combat drug-resistant pathogens are being developed but may not be readily available (12). In the United States, prolonged hospitalization and the use of more expensive antimicrobial agents have increased the cost of treating resistant infections, which is estimated to range from $100 million to $30 billion per year (13). Many of these issues were addressed in a 1992 report by the Institute of Medicine, Emerging Infections: Microbial Threats to Health in the United States, which emphasized the need for improved surveillance and control of pathogens that are resistant to antimicrobial drugs (14). Although as of December 1995, 13 states and one city health department have made mandatory the reporting of DRSP, no coordinated national effort has been initiated previously to address this problem. The spread of DRSP presents a challenge to clinicians, laboratorians, and public health practitioners to identify and implement prevention and control methods to minimize the complications of DRSP infections (e.g., greater duration and severity of illness, health-care expenses, and mortality). In June 1994, a CDC-convened working group of public health practitioners, clinical laboratorians, health-care providers, and representatives of key professional societies identified the development of a nationwide laboratory-based surveillance system as the essential first step in a strategy of surveillance, investigation, prevention, and control of DRSP. The strategy is intended to be flexible and may change as a result of data obtained during initial phases of its implementation and from new studies. This report describes the three goals developed by the working group -- surveillance, epidemiologic investigation, and prevention and control of DRSP -- and the objectives for each goal. GOALS AND OBJECTIVES Goal I. Surveillance: define and monitor the prevalence and geographic distribution of DRSP and rapidly recognize the emergence of new patterns of resistance. The incidence of penicillin-resistant S. pneumoniae has increased in certain sentinel sites in the United States (1). These sites detected a 60-fold increase in high-level resistance to penicillin among isolates from several large hospitals located primarily in urban areas; however, these data are not representative of many communities in other parts of the country. Resistance to antimicrobial drugs appears to vary among communities and even among hospitals in the same city. Prevention or control programs designed to address increasing and variable resistance to antimicrobial drugs should include surveillance to detect levels of resistance specific to different communities. Objective A. Establish nationwide mandatory reporting of DRSP. Concern about increasing resistance to antimicrobial agents has prompted 13 state health departments (Arkansas, Colorado, Connecticut, Georgia, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New York, North Carolina, Ohio, and South Carolina) and the New York City Health Department to institute regulations requiring laboratories to report DRSP isolates from certain anatomic sites (e.g., cerebrospinal fluid {CSF} and blood). To ensure that such a surveillance system has maximum participation and more nationally representative data, a nationwide requirement for reporting DRSP isolates is needed. In late 1994, the DRSP working group submitted a proposal to the Council of State and Territorial Epidemiologists (CSTE) that required all states to report invasive infections caused by DRSP. This proposal was approved by CSTE in January 1995 (15). Although regulatory authority for reporting nationally notifiable diseases resides at the state level, approval by CSTE provides a basis for state health officials to encourage their state legislators to adopt the measure. Objective B. Improve the detection of DRSP in laboratories by promoting appropriate interpretive standards for identification and susceptibility testing of S. pneumoniae. On the basis of National Committee for Clinical Laboratory Standards (NCCLS) interpretive standards, all isolates of S. pneumoniae from usually sterile sites should be tested for penicillin resistance (16). Pneumococcal resistance to penicillin can be screened initially by using a 1 ug oxacillin disk; penicillin resistance is considered probable with oxacillin zone size less than or equal to 19 mm. The screening approach is highly sensitive (99%) and specific (80%-90%) and should detect almost all isolates resistant to penicillin and extended-spectrum cephalosporins (e.g., ceftriaxone or cefotaxime). Isolates found to be nonsusceptible by oxacillin disk should then be subjected to quantitative MIC testing against penicillin, an extended-spectrum cephalosporin, chloramphenicol, vancomycin, and other drugs clinically indicated to treat the patient. MIC testing should be performed by using methods determined by NCCLS to be valid and reliable (e.g., broth microdilution, agar dilution, disk diffusion, or antimicrobial gradient strips). A recent CDC survey of laboratories providing service to large academic pediatric centers indicated that 85% of these laboratories were screening pneumococcal isolates for penicillin resistance by using a 1 ug oxacillin disk, a method recommended by NCCLS (CDC, unpublished data). Data from a statewide survey performed by the Colorado State Department of Health demonstrated that of 78 laboratories surveyed, 57 (73%) were using oxacillin screening for S. pneumoniae isolates for penicillin susceptibility; 29 (37%) were screening all isolates. A survey from the New York City Department of Health demonstrated that 68% of hospital and commercial laboratories in New York City screened with oxacillin testing, either all isolates or all sterile site isolates. The degree to which smaller hospital laboratories in other areas of the country adhere to the NCCLS interpretive standards for pneumococcal testing is less well known. Many laboratories also are in periods of transition while implementing methods newly approved by NCCLS, such as changes in interpretive breakpoints (e.g., zone diameters) and recommendations against the use of automated methods of minimal inhibitory concentration (MIC) susceptibility testing for S. pneumoniae (17,18). The current mechanism for disseminating information from NCCLS to laboratories should be augmented to reach the majority of laboratorians and health-care providers. Thus, distribution should be expanded by publishing NCCLS's current recommendations on routine testing and reporting of S. pneumoniae and interpretative standards in publications likely to be received by laboratorians and health-care providers ((Table_1) (Table_2) (Table_3)). In addition, the working group has asked selected researchers to submit letters to appropriate laboratory-oriented publications encouraging the prompt institution of NCCLS interpretive standards for pneumococcal testing. The published recommendations should stress prompt adherence to NCCLS interpretive standards and promote the use of appropriate methods for MIC testing. The interpretive standards are included in this report to disseminate the most current recommendations concerning MIC interpretive breakpoints ((Table_1) (Table_2) (Table_3)). Additionally, the CDC electronic surveillance module for capturing DRSP prevalence data (DRSP-PHLIS {Public Health Laboratory Information System}, v. 3.1) has incorporated the 1994 NCCLS interpretive breakpoints into the computer menu to assist users of this surveillance system with consistent interpretation of MIC data. Clinical laboratorians who suspect a pneumococcal isolate to be nonsusceptible to vancomycin should a) verify the results, b) save the isolate, and c) report the confirmed results to the respective state health department and to CDC (Childhood and Respiratory Diseases Branch, Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases). Objective C. Develop an electronic, laboratory-based surveillance system capable of reporting DRSP and other conditions. Surveillance for pneumococcal disease has been limited in the past. Since 1979, CDC has been actively monitoring trends in invasive pneumococcal disease at 13 sentinel sites nationwide. In addition, CDC-funded, population-based surveillance projects have been under development since October 1994 to monitor pneumococcal disease at selected locations in several states (i.e., California, Connecticut, Georgia, Maryland, Minnesota, Oregon, Tennessee, and Texas). Researchers at academic medical centers have gathered data on DRSP and other antimicrobial-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE). Although these activities have yielded useful information regarding the incidence of DRSP, they reflect the levels of resistance specific to those sites. Geographic variation in resistance to antimicrobial drugs among communities and among hospitals in the same community is common. Population-based laboratory surveillance is necessary to reflect accurately local geographic and temporal trends in DRSP. The surveillance system should capture data from as many laboratories as possible within each community. These data must be aggregated, analyzed, and reported to local health-care providers in a timely manner. Clinicians need DRSP prevalence data specific to their community to select appropriate antimicrobial agents when empirically treating persons who have pneumococcal infections. For example, in communities that have a high incidence of DRSP, persons who have life-threatening infections might receive therapy with vancomycin in combination with an extended-spectrum cephalosporin until results of CSF or blood cultures are known. Additionally, in areas known to have low levels of pneumococci resistant to extended-spectrum cephalosporins, empiric vancomycin use can be avoided, thereby reducing overuse of an important antimicrobial drug. Rapid increases in pneumococcal resistance patterns have occurred over a short time in many communities; therefore, recommendations regarding empiric therapy must be based on the most recent data available. Public health practitioners also will benefit from knowledge of communitywide trends in pneumococcal resistance. For example, if such practitioners identify areas within their jurisdictions with high levels of resistance requiring more immediate control measures, efforts can then be directed toward increasing vaccine use in these communities to prevent illness in persons who are at high risk for pneumococcal illness. These vaccination efforts should be part of a broader campaign to increase vaccine use in general. Laboratory surveillance for invasive pneumococcal isolates and their susceptibility patterns can be used also to monitor changes in pneumococcal incidence after the introduction of protein conjugate pneumococcal vaccines that are now being developed for use in children and adults. To decrease the burden (e.g., time, staffing, and other resources) to laboratory personnel, any system for laboratory-based reporting should use existing computerized data that might be already stored electronically in a laboratory information system (LIS). In addition, reporting software should be flexible enough to receive and manage data for multiple laboratory-reportable conditions (e.g., Haemophilus influenzae type B and Neisseria meningitidis). A laboratory-based surveillance system developed for DRSP should use electronic data management and transfer methods when appropriate and allow for feedback of information to the laboratory, state and local health departments, CDC, and health-care professionals (Figure_1). The software and the methods of data transfer chosen for electronic reporting should be expandable and standardized and should allow for reporting conditions other than DRSP. A proposed electronic, laboratory-based system is described in this report (Appendix). Goal II. Investigation: improve the understanding of the epidemiology and clinical impact of DRSP. Objective A. Identify risk factors for development of resistance to antimicrobial drugs by conducting epidemiologic investigations in communities that have unusually high or low levels of DRSP. Levels of pneumococcal antimicrobial resistance can vary among communities. Identifying regions that have unusually high or low levels of resistance, as well as identifying outbreaks caused by resistant strains, will assist investigators in more accurately characterizing the epidemiology of DRSP and in developing methods to prevent and control the complications of DRSP-related illness. Molecular methods will be employed to track epidemiologically the spread of highly resistant strains and to gain further understanding of mechanisms of resistance to antimicrobial drugs. By focusing on communities that have unusually high levels of resistance to antimicrobial drugs, investigators can evaluate risk factors that promote the development of antimicrobial resistance. CDC and state and local health departments also should focus investigations on communities with low levels of resistance to antimicrobial drugs to identify factors that have deterred the increase of antimicrobial resistance and to implement programs for preventing the spread of DRSP. Comparing communities with high and low levels of resistance can help to identify risk factors for antimicrobial resistance. Studies are currently being conducted in several cities to identify risk factors for development of DRSP and for increased morbidity from these infections. Objective B. Determine outcome and treatment costs of DRSP infections and formulate recommendations for changing empiric antimicrobial therapy on the basis of community-specific levels of resistance to antimicrobial drugs. CDC studies are under way to determine the cost of treatment and outcome of invasive DRSP infections. This information will be used to develop models that public health officials at the state and local levels can use to determine thresholds for recommending changes in antimicrobial therapy. Because initial therapy for pneumococcal infection is usually empiric, the selection of antimicrobial agents should be guided by the regional prevalence of DRSP. Surveillance for DRSP will provide data on community-specific levels of resistance. These data can be useful in deciding when to change empiric therapy. Although no consensus exists for this decision making, many factors (e.g., outcome of infection, cost of treating infection, susceptibility of the population, and access to medical care) may be important for determining a threshold at which empiric therapy should be changed. Through the process of decision analysis, these factors can be incorporated into a model that can be used for determining the community-specific proportion of resistant pneumococcal isolates at which a change in empiric therapy should be recommended to clinicians in the community. Objective C. Investigate the transmission of antimicrobial-resistant pneumococci through studies of nasopharyngeal colonization. Several factors have been associated with the emergence of DRSP, most notably attendance in child day care centers and frequent use of antimicrobial drugs. Because many earlier studies of pneumococcal transmission were performed before widespread emergence of penicillin resistance, the relevance of these findings for DRSP is not clear, and several issues regarding the transmission of DRSP remain to be examined. Identifying these key factors for transmission will help to improve prevention and control strategies. To determine the association of nasopharyngeal carriage of DRSP with development of invasive DRSP infection, several studies recently have been initiated in Georgia, Maryland, and Texas. The studies are intended to provide information on patterns of resistance and proportions of DRSP nasopharyngeal isolates in the community and their relation to invasive DRSP isolates identified during communitywide surveillance. If isolates identified through nasopharyngeal cultures are representative of isolates that cause invasive disease, surveillance of DRSP by using nasopharyngeal cultures may be useful in areas where laboratory-based surveillance is not possible. For small population areas, long periods of time may be required to obtain sufficient numbers of cases of invasive pneumococcal isolates before precise estimates of DRSP prevalence can be obtained. In these communities, surveillance by using nasopharyngeal cultures might provide a more rapid estimate of the prevalence of DRSP. Goal III. Prevention and control: minimize complications of DRSP infections through prevention and control of DRSP infections. Objective A. Improve vaccination use nationally, targeting areas most likely to benefit from intervention (e.g., regions with high levels of resistance to antimicrobial drugs or communities of persons at highest risk for infection). A vaccine for the 23 most common serotypes of S. pneumoniae is available, yet underutilized. Healthy People 2000 objectives target the vaccination of 60% of persons at risk for pneumococcal illness by the year 2000 (19). As of 1993, data from the National Health Interview Survey indicated that only 27% of persons greater than or equal to 65 years of age had been vaccinated (4). Reasons for insufficient use of the vaccine differ; however, vaccine use increases when efforts are made to raise awareness and promote the benefits of vaccination. Communities with high levels of antimicrobial resistance and persons at highest risk for infection will benefit from targeted campaigns to increase rates of vaccination. The 23-valent pneumococcal vaccine is not adequately immunogenic in young children; thus, it is not recommended for use in children less than 2 years of age, a population at high risk for acute pneumococcal otitis media, bacteremia, and meningitis. In conjunction with state health departments and the Health Care Financing Administration, CDC is developing and expanding programs to promote the use of pneumococcal vaccine. By using data obtained from surveillance for DRSP, state and local health departments can target areas of high-level pneumococcal antimicrobial resistance for increased vaccination. Promotions for vaccination among the general public and for targeted groups can be disseminated through media releases, medical societies, infection-control practitioners, and health-care providers (e.g., health maintenance organizations, hospitals, and health clinics). An effective pneumococcal protein-conjugate vaccine prescribed at least for all children less than 2 years of age would be the most effective means of preventing pneumococcal illness in children; however, conjugate vaccines are still being developed and evaluated. If effective, approved vaccines will likely not be available for several years. As surveillance for DRSP improves, the prevalence of antimicrobial resistance may reach levels sufficiently high to warrant changes in the current recommendations for pneumococcal polysaccharide vaccine use. Such necessary changes may include reexamining the definition of persons at high risk for pneumococcal infection to increase vaccine use (e.g., by children in day care centers). Objective B. Promote the judicious use of antimicrobial agents. Increased use of antimicrobial drugs encourages the spread of resistance and increases the prevalence of drug-resistant strains. Data from hospital-based studies indicate an association between increased rates of use of antimicrobial agents and resistant nosocomial infections (20); data from studies associating rates of use of antimicrobial agents and resistance on a district or national scale indicate a similar association (21,22). In addition, multiple case-control studies have indicated that previous use of antimicrobial drugs is a substantial risk factor for infection with a resistant pathogen (2,23). Most antimicrobial drugs are administered for treatment of outpatient infections. In 1992, in the United States, an estimated 110 million courses of antimicrobial therapy were prescribed by office-based physicians -- a 28% increase over the number prescribed in 1980 (24). Rates of use of antimicrobial agents for children less than 15 years of age were approximately three times greater than those for other age groups. In 1990, common respiratory infections, upper respiratory tract infection, bronchitis, sinusitis, and pharyngitis were the five leading diagnoses resulting in prescriptions for antimicrobial drugs. Although appropriate antimicrobial-drug use has unquestioned benefit, often these agents are used inappropriately by physicians and the public. The following practices of physicians may contribute to inappropriate use of antimicrobial drugs: Providing antibacterial drugs to treat viral infections. In 1992, upper respiratory tract infections were the second leading indication for outpatient use of antibacterial drugs (24). Studies have demonstrated that antibacterial therapy for nonspecific upper respiratory infections does not benefit patients because these infections have a viral etiology but does increase the likelihood that resistant organisms will be selected. Using inadequate diagnostic criteria for infections that might have a bacterial etiology. Patients who have a cough and nasal discharge frequently are diagnosed as having bronchitis and sinusitis -- the third and fifth leading diagnoses resulting in outpatient antimicrobial use (24). However, the specificity of these diagnoses for an infection that truly requires treatment will be low unless appropriate criteria are used in making the diagnosis. Establishing age-appropriate standards for diagnosing these conditions would assist physicians in deciding which patients require antimicrobial therapy and which patients can be sent home with appropriate follow-up and no antimicrobial drugs. For pharyngitis -- the fourth leading diagnosis -- standards for diagnosis already have been established by the Committee on Infectious Diseases of the American Academy of Pediatrics (24,25); however, these guidelines frequently are not followed (26). Prescribing expensive, broad-spectrum agents that are unnecessary, determining improper dose and duration of therapy, and not following established recommendations for chemoprophylaxis. Although amoxicillin remains an effective first-line therapy for most children who have otitis media, data from a recent study among children in a managed-care organization demonstrated that during 1993-1994, 24% and 17% of children with otitis media had been treated with cephalosporins and combination agents, respectively (CDC, unpublished data). Drugs used for otitis media prophylaxis included cephalosporins as well as the recommended agents amoxicillin and sulfisoxazole (25). Reasons for overuse of antimicrobial drugs are manifold. Physicians may be uncertain regarding the optimal approach to diagnosis and the best therapy for an individual patient, particularly in a community where antimicrobial resistance already is present. Practices concerning the use of antimicrobial drugs may also be affected by physicians' concerns regarding not treating infections potentially caused by bacteria and the possibility of subsequent lawsuits from untoward outcomes. Patients and parents contribute to the misuse of antimicrobial drugs by pressuring physicians to provide treatment. Developing effective strategies to improve antimicrobial use will require the recognition that the physician and the patient (or the patient's parent{s}) are partners in health care and that information must be provided to both groups. These strategies should ensure that bacterial infections are appropriately treated in terms of health outcome and cost, while preserving the usefulness of antimicrobial agents in treating future disease. Beginning in 1995, CDC, in collaboration with professional societies, managed-care organizations, and other groups, initiated projects to a) evaluate current knowledge, attitudes, and behaviors of physicians and the public regarding use of antimicrobial agents; b) develop standards for diagnosis, therapy, and prophylaxis of common outpatient respiratory tract infections; c) develop educational materials for providers and patients/parents by promoting appropriate diagnostic methods and judicious antimicrobial use; and d) evaluate the impact of these interventions. Although changing behaviors regarding the use of antimicrobial drugs will require a long-term, multifaceted strategy, these projects will be an important first step in reaching Objective B. Objective C. Establish rational treatment guidelines for use by physicians treating presumptive pneumococcal infections. Relevant clinical interpretations of data pertaining to antimicrobial resistance obtained through the proposed surveillance system should be communicated to clinicians treating patients who have presumed pneumococcal illness. Physicians in communities that have high levels of antimicrobial resistance should be informed of appropriate empiric choices of antimicrobial agents. Those in areas that have low levels of resistance also should be informed so that unnecessary drug combinations or inappropriately broad regimens are not used. This information, which constitutes the feedback arm of the surveillance system, should be easily accessible to public health officials and provided regularly to clinicians. To establish treatment guidelines, persons from the DRSP working group, professional societies, academic institutions, state health agencies, and CDC will examine available data and work to develop recommendations that represent a rational approach to antimicrobial therapy in the context of increasing antimicrobial resistance. These guidelines should be used to assist state and local health departments in recommending appropriate empiric antimicrobial regimens and vaccination strategies based on susceptibility trends in their jurisdictions. Medical societies, communicable-disease newsletters, hospitals, and managed-care organizations may be useful resources for sharing information. Because rapid changes in resistance levels can occur, surveillance data and recommendations should be communicated to clinicians either monthly or bimonthly. Objective D. Periodically publish national and regional trends in pneumococcal antimicrobial resistance. Data from the proposed surveillance system will be received at CDC from various state health departments as described previously in Goal I, Objective C. These data will be analyzed to develop a national profile of DRSP incidence showing geographic patterns of pneumococcal resistance. The results of data analyses will be used by health-care providers, public health officials, and managed-care organizations to develop policies regarding DRSP and to develop treatment guidelines. These data also may give manufacturers of antimicrobial drugs more accurate descriptions of the antimicrobial susceptibilities of drugs used to treat pneumococcal infections. Early in the implementation of the surveillance system, CDC will publish DRSP incidence data quarterly in the MMWR (weekly) indicating individual state totals and national trends. The data also will be disseminated periodically in appropriate journals. New regulations from the Food and Drug Administration may require package inserts for antimicrobial drugs to contain information on antimicrobial susceptibility obtained from national surveillance databases. CONCLUSIONS The DRSP working group's strategy for surveillance, investigation, prevention, and control of infections caused by DRSP has focused on implementation of a laboratory-based, electronic surveillance system for reporting invasive DRSP infections, increased pneumococcal vaccination, and promotions of judicious use of antimicrobial drugs. Data received through the surveillance system will be used to determine community-specific levels of pneumococcal antimicrobial resistance. This information will be made available to health-care providers and clinicians to promote appropriate use of antimicrobial drugs and increased vaccine use. The intended outcome of the surveillance system is to control the spread of DRSP and to minimize complications of DRSP infection (e.g., greater duration and severity of illness, long-term sequelae, health-care expenditures, and mortality). The strategy is intended to be flexible and may change on the basis of information obtained during initial phases of its implementation and on results of new studies. References
Schwartz B, Fries S, Fitzgibbon AM, Lipman H. Pediatricians'
diagnostic approach to pharyngitis and impact of CLIA 1988 on
office diagnostic tests. JAMA 1994;271:234-8.
Table_1
TABLE 1. Suggested groupings of approved antimicrobial agents * that
should be considered for routine testing and reporting of Streptococcus
pneumoniae by clinical microbiology laboratories +
===============================================================================
Groupings Antimicrobial agents
-------------------------------------------------------------------------------
Group A: Primary test and report Penicillin (oxacillin disk)
Erythromycin & @
Trimethoprim/sulfamethoxazole &
Group B: Primary test, report selectively Vancomycin
Tetracycline &
Chloramphenicol
Group C: Supplemental, report selectively Ofloxacin
-------------------------------------------------------------------------------
* Approved by the Food and Drug Administration.
+ Adapted with permission from the National Committee for Clinical Laboratory
Standards (NCCLS). Performance standards for antimicrobial susceptibility
testing (fifth informational supplement). Villanova, PA: NCCLS, 1994; NCCLS
document vol. 14, no. 16, M100-S5, M7-A3, Table 1A.
& Only results of testing with penicillin, chloramphenicol, vancomycin, and
cefotaxime or ceftriaxone (if tested by a dilution method as outlined in
NCCLS document M7 of the fifth informational supplement vol. 14, no. 16,
1994) should be reported routinely with blood and CSF isolates of S.
pneumoniae recovered from patients who have life-threatening infections
(e.g., meningitis and bacteremia).
@ Susceptibility and resistance to azithromycin and clarithromycin can be
predicted by susceptibility testing of erythromycin.
NOTE: Selection of the most appropriate antimicrobial agents to test and
report is a decision best made by each clinical laboratory in consultation
with infectious disease practitioners, the pharmacy, and the pharmacy and
infection-control committees of the medical staff. The antimicrobial agents
listed above in each grouping have, during in vitro tests, demonstrated
acceptable efficacy. Considerations in the assignment of agents to Groups
A, B, and C include clinical efficacy, prevalence of resistance, minimizing
emergence of resistance, cost, and current consensus recommendations for
first choice and alternative drugs. Tests on selected agents may be useful
for infection control.
===============================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Zone diameter interpretive standards and equivalent minimum inhibitory concentration
(MIC) breakpoints for Streptococcus pneumoniae *
==================================================================================================
Disk Zone diameter + Equivalent MIC
Antimicrobial content (nearest whole mm) breakpoints & (ug/mL)
agent (ug) Resistant Intermediate Susceptible Resistant Susceptible
--------------------------------------------------------------------------------------------------
Azithromycin @ 15 <=13 14-17 >=18 >=2 <=0.50
Chloramphenicol 30 <=20 - >=21 >=8 <=4.00
Clarithromycin @ 15 <=16 17-20 >=21 >=2 <=0.50
Clindamycin @ 2 <=15 16-18 >=19 >=1 <=0.25
Erythromycin 15 <=15 16-20 >=21 >=4 <=0.50
Ofloxacin 5 <=12 13-15 >=16 >=8 <=2.00
Penicillin
(oxacillin disk) ** 1 - - >=20 - <=0.06
Rifampin @ 5 <=16 17-18 >=19 >=4 <=1.00
Tetracycline 30 <=17 18-21 >=22 >=8 <=2.00
Trimethoprim/
sulfamethoxazole 1.25/23.75 <=15 16-18 >=19 >=4/76 <=0.50/9.50
Vancomycin 30 - - >=17 - <=1.00
--------------------------------------------------------------------------------------------------
* Adapted with permission from the National Committee for Clinical Laboratory Standards (NCCLS).
Performance standards for antimicrobial susceptibility testing (fifth informational supplement).
Villanova, PA: NCCLS, 1994; NCCLS document vol. 14, no. 16, M100-S5, M2-A5, Table 2C.
+ These zone diameter standards apply only to tests performed by using Mueller-Hinton agar
supplemented with 5% sheep blood.
& These values represent MIC breakpoints used in determining approximate zone size interpretive
criteria. They relate to MICs determined by M7 methodology. Equivalent MIC breakpoints relate
to tests performed by broth microdilution using cation-adjusted Mueller-Hinton Broth with
2%-5% lysed horse blood.
@ Zone diameters and MIC breakpoints are considered tentative for 1995.
** Isolates of pneumococci with oxacillin zone sizes of >=20mm are susceptible (MIC <=0.06 ug/mL)
to penicillin and can be considered susceptible to ampicillin, amoxicillin,
amoxicillin/clavulanic acid, ampicillin/sulbactam, cefaclor, cefepime, cefetamet, cefixime,
cefotaxime, cefprozil, ceftibuten, ceftriaxone, cefuroxime, cefpodoxime, ceftizoxime,
imipenem, and loracarbef for approved indications, and these agents need not be tested.
A penicillin MIC should be determined on isolates of S. pneumoniae with oxacillin zone sizes
of <=19mm. The disk test does not distinguish penicillin intermediate strains (i.e.,
MICs=0.12-1.0 ug/mL) from strains that are penicillin resistant (i.e., MICs >=2.0 ug/mL).
Reliable disk diffusion tests for cefotaxime and ceftriaxone do not yet exist; their in vitro
activity is best assessed by using an MIC method. The absence of resistant strains precludes
defining any results categories other than susceptible. If clinical laboratorians
suspect a pneumococcal isolate to be nonsusceptible to vancomycin, they should 1) verify the
reports, 2) save the isolate, and 3) report the confirmed result to the respective state
health department and to CDC.
==================================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 3. Minimum inhibitory concentration (MIC) interpretive standards (ug/mL) for Streptococcus pneumoniae * + ======================================================================= Antimicrobial agent Susceptible Intermediate Resistant ----------------------------------------------------------------------- Azithromycin <=0.50 1.00 >=2.00 Cefotaxime & <=0.50 1.00 >=2.00 Cefepime <=0.50 1.00 >=2.00 Ceftriaxone & <=0.50 1.00 >=2.00 Cefuroxime axetil (oral) <=0.50 1.00 >=2.00 Chloramphenicol <=4.00 - >=8.00 Clarithromycin <=0.50 1.00 >=2.00 Clindamycin <=0.25 0.50 >=1.00 Erythromycin <=0.50 1.00-2.00 >=4.00 Imipenem <=0.12 0.25-0.50 >=1.00 Ofloxacin <=2.00 4.00 >=8.00 Penicillin @ <=0.06 0.10-1.00 >=2.00 Rifampin <=1.00 2.00 >=4.00 Tetracycline <=2.00 4.00 >=8.00 Trimethoprim/ sulfamethoxazole <=0.50/9.50 1.00/19.00-2.00/38.00 >=4/76 Vancomycin ** <=1.00 - - ----------------------------------------------------------------------- * Adapted with permission from the National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial susceptibility testing (fifth informational supplement). Villanova, PA: NCCLS, 1994; NCCLS document vol. 14, no. 16, M100-S5, M7-A3, Table 2C. + These interpretive standards are applicable to only broth microdilution susceptibility tests with S. pneumoniae using cation-adjusted Mueller-Hinton broth with 2%-5% lysed horse blood. & When recovered from patients who have meningitis, strains in the intermediate category may require therapy with maximum doses of the drug. @ A pneumococcal isolate that is susceptible to penicillin can be considered susceptible to ampicillin, amoxicillin, amoxicillin/clavulanic acid, ampicillin/sulbactam, cefaclor, cefepime, cefetamet, cefixime, cefotaxime, cefprozil, ceftibuten, ceftriaxone, cefuroxime, cefpodoxime, ceftizoxime, imipenem, and loracarbef for approved indications. Testing of these agents (except cefepime, cefotaxime, ceftriaxone, or cefuroxime axetil) against penicillin-intermediate or penicillin-resistant isolates is not recommended. Currently, reliable interpretive criteria for these agents are not available. Physicians should be informed that clinical response rates with these agents may be lower in strains that are not susceptible to penicillin. ** The absence of resistant strains precludes defining any results categories other than susceptible. If clinical laboratorians suspect a pneumococcal isolate to be nonsusceptible to vancomycin, they should 1) verify the reports, 2) save the isolate, and 3) report the confirmed result to the respective state health department and to CDC. ======================================================================= Return to top. Figure_1 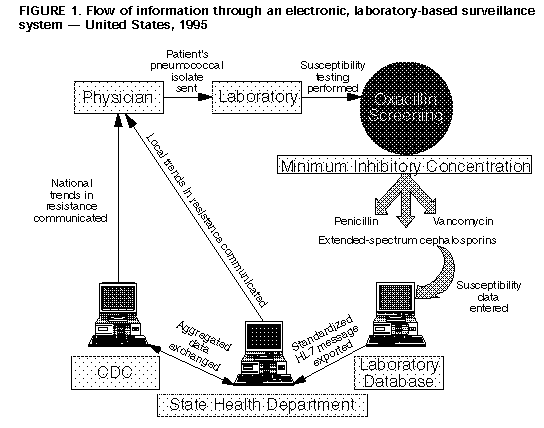 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|