 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Paralytic Poliomyelitis -- United States, 1980-1994The Advisory Committee on Immunization Practices (ACIP) recently recommended a sequential vaccination schedule of two doses of inactivated poliovirus vaccine (IPV) followed by two doses of oral poliovirus vaccine (OPV) for routine vaccination of children in the United States (1). ACIP revised its recommendation for routine poliovirus vaccination for three reasons: 1) paralytic poliomyelitis attributable to indigenously acquired wild poliovirus has not occurred in the United States since 1979 (2), 2) progress toward global eradication of poliomyelitis has reduced the risk for importation of wild poliovirus into the United States (3), and 3) vaccine-associated paralytic poliomyelitis (VAPP) continues to occur. ACIP has recommended that implementation of this new vaccination schedule begin in early 1997. This report summarizes both the epidemiology of paralytic poliomyelitis in the United States reported during 1980-1994 and provisional reports for 1995-1996 and updates the estimated risk for VAPP. These findings indicate that the overall estimated risk for VAPP has remained constant. Epidemiology During 1980-1994, state and territorial health departments reported to CDC 133 confirmed cases of paralytic poliomyelitis. Of these, 125 (94%) cases were associated with administration of OPV (annual mean: eight VAPP cases) (Figure_1); six cases (only one of which occurred after 1986) were classified as imported; and two were classified as indeterminate (no poliovirus was isolated from samples obtained from the patients, and these persons had no history of recent vaccination or direct contact with a vaccine recipient). Of the 125 VAPP cases, 49 (39%) occurred among immunologically normal recipients of OPV, 46 (37%) among immunologically normal contacts of OPV recipients (including six cases among persons from whom vaccine-like poliovirus was isolated but who had no history of direct contact with vaccinees), and 30 (24%) among immunologically compromised OPV recipients or contacts of OPV recipients. Provisional reports include an additional six confirmed cases for 1995 and one confirmed case for 1996. During 1980-1994, of the 125 VAPP cases, 97 (78%) were associated with administration of the first or second dose of OPV. Of the 49 cases among immunologically normal OPV recipients (Table_1), 45 (92%) were associated with administration of the first or second dose of OPV, and 41 (91%) of these were among persons aged less than 1 year. Of the 46 cases among immunologically normal contacts, 32 (70%) were associated with the first or second dose of OPV, and 26 (81%) of these occurred among persons aged greater than or equal to 20 years. Of the 23 cases among immunologically compromised vaccine recipients, 15 (65%) were associated with administration of the first or second dose of OPV, and 12 (80%) of these were among persons aged less than 1 year. Of the seven cases among immunologically compromised contacts, five (71%) were associated with administration of the first or second dose, and four (80%) of these occurred among persons aged greater than or equal to 20 years. Of the immunologically compromised vaccine recipients with VAPP, none had immunodeficiency diagnosed before onset of paralysis. Of the seven persons with cases of VAPP during 1995-1996, five were OPV recipients, and all of these were aged less than 1 year. To analyze temporal trends, cases of VAPP among OPV recipients and contacts were grouped by 3-year intervals from 1980 through 1994. The number of cases among all vaccine recipients occurring during each 3-year period remained relatively constant (range: 13-16, p=0.71), and there were no substantial temporal changes in the number of cases among immunologically normal recipients (p=0.18). However, from 1980-1982 to 1992-1994, the number of cases among contacts declined significantly, from 15 to four (p=0.01) (Table_1). Estimated Risks for VAPP Based on the distribution of an estimated 303 million total doses of OPV during 1980-1994, the overall risk (as measured by a ratio) for VAPP during this period was one case to the 2.4 million doses distributed; for children receiving their first doses of OPV, the ratio was one case to the 750,000 children. Assuming that all 57.8 million children born during 1980-1994 received a first dose of OPV, the ratio for immunologically normal first-dose recipients was one case to the 1.4 million first doses distributed. For all first-dose recipients (immunologically normal or compromised), the ratio was one case to the 1.2 million first doses distributed. Based on all doses distributed, the ratio for recipients was one case to the 6.2 million doses distributed. Samples for poliovirus isolation were obtained from 110 (88%) of the 125 persons with VAPP; of these, poliovirus was isolated from the samples from 89 (81%) persons. Of the 109 cases for which the date of sample collection was known, poliovirus was isolated from 83 (85%) of the 98 samples obtained less than or equal to 15 days after onset of paralysis and from five (46%) of the 11 samples obtained greater than 15 days after onset of paralysis. Cases were reported to CDC a median of 47 days (range: 1 day-13 years) after onset of paralysis. Reported by: Child Vaccine Preventable Disease Br, Epidemiology and Surveillance Div, National Immunization Program, CDC. Editorial NoteEditorial Note: The new sequential IPV/OPV poliovirus vaccination schedule is expected to reduce the incidence of VAPP while maintaining individual and population immunity against polioviruses at the high levels necessary to prevent polio outbreaks if wild poliovirus is reintroduced into the United States. Use of IPV as the first two doses of the sequential vaccination schedule should induce high levels of protective antibodies among immunologically normal vaccine recipients by the time the first dose of OPV is administered at age 12-18 months, thereby eliminating 95% of VAPP cases among these children (4). In addition, the number of contact cases may decrease because of reduced transmission (attributed to some IPV-induced immunity of pharyngeal mucosa and, to a lesser degree, of intestinal mucosa) of vaccine virus from persons given OPV after receipt of two doses of IPV. Delaying administration of the first dose of OPV until age 12 months may allow additional time for diagnosis of immunodeficiency (which contraindicates receipt of OPV) and enable prevention of some VAPP cases among immunodeficient recipients (4,5). The overall estimated risk for VAPP in the United States has remained relatively constant since 1965 (2,6,7); however, the findings in this report suggest that the risk for VAPP among contacts of OPV recipients has decreased since 1980. Because of reporting delays, the data for recent years may not be complete, and additional years of observation are required to confirm this trend. Although reasons for the decline have not been firmly established, enforcement of state vaccination requirements for school entry beginning in the late 1960s may have decreased the proportion of parents and adult close contacts of vaccinees without immunity to poliovirus, particularly in areas where compliance with these requirements is high (8). However, other reports suggest that the prevalence of poliovirus susceptibility among young adults did not change during this period: for example, data from a national serosurvey of U.S. Army recruits born during 1954-1972 indicated that 12.5%-13.0% were susceptible to poliovirus type 3, and that no statistically significant decline in the percentage susceptible occurred among recruits born after 1966 (9). Enhanced surveillance for paralytic poliomyelitis (whether vaccine- or wild poliovirus-associated) is necessary to monitor the impact of the change in the routine poliovirus vaccination policy. The completeness of reporting for diagnosed paralytic poliomyelitis in the United States has been estimated at 81%. Misdiagnosis or failure to report diagnosed cases contributes to incomplete surveillance (6). Any suspected case of paralytic poliomyelitis must be reported immediately to state or local health authorities (10). A clinical case definition for reporting cases of paralytic poliomyelitis * was adopted by the Council of State and Territorial Epidemiologists in 1990. Confirmed cases are those that meet the clinical case definition and in which the patient has a neurologic deficit 60 days after onset of initial symptoms, has died, or has unknown follow-up status. Experts at state and local health departments and at CDC are available for consultation about patients who have suspected cases of paralytic poliomyelitis (i.e., patients with acute paralytic manifestations), and information about clinical diagnosis and reporting of cases is available from CDC's National Immunization Program, telephone (404) 639-8255. Laboratory studies, especially attempted poliovirus isolation, are critical for ruling out or confirming paralytic poliomyelitis. Specimens for virus isolation (e.g., stool, throat swab, and cerebrospinal fluid) and serologic testing must be obtained in a timely manner. At least two stool specimens and two throat swabs should be obtained as early as possible in the course of illness (optimally within 15 days of onset) from patients who are suspected to have polio. Cultures for enterovirus followed by enteroviral typing should be considered for all patients with acute flaccid paralysis unless an alternative diagnosis is apparent. Intratypic differentiation must be performed to determine whether a poliovirus isolate is vaccine-related or wild type. The Enterovirus Laboratory of CDC's National Center for Infectious Diseases is the national reference laboratory for polioviruses in the United States and the only laboratory that performs this procedure on a routine basis. Information about collection and shipment of clinical specimens is available from this laboratory, telephone (404) 639-2749. References
* Acute onset of flaccid paralysis in one or more limbs with decreased or absent tendon reflexes in the affected limbs without other apparent cause and without sensory or cognitive loss (as reported by a physician). Figure_1 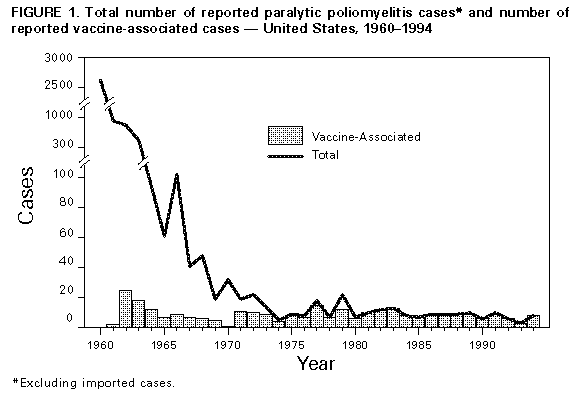 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Number of cases of vaccine-associated paralytic poliomyelitis (VAPP) among recipients of oral
poliovirus vaccine (OPV) and contacts of OPV recipients, by 3-year groupings -- United States, 1980-1994
============================================================================================================
VAPP cases
----------------------------------------------------------------------------
Category 1980-1982 1983-1985 1986-1988 1989-1991 1992-1994 * Total
------------------------------------------------------------------------------------------------------------
Recipients
Immunologically normal 10 12 11 8 8 49
Immunologically compromised 3 2 5 8 5 23
Total 13 14 16 16 13 72
Contacts
Immunologically normal 14 12 9 7 4 46
Immunologically compromised 1 2 2 2 0 7
Total 15 14 11 9 4 53
Total 28 28 27 25 17 125
------------------------------------------------------------------------------------------------------------
* Because of late reporting, the number of cases during this period may be incomplete.
============================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|