 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- Bangladesh, 1995-1997As a member of the World Health Assembly, in 1988 Bangladesh adopted the goal of poliomyelitis eradication by 2000 (1). To achieve this goal, Bangladesh has implemented the following strategies recommended by the World Health Organization (WHO): 1) achieving high routine coverage with at least three doses of oral poliovirus vaccine (OPV3) among infants aged less than 12 months; 2) conducting National Immunization Days (NIDs)* to interrupt widespread circulation of poliovirus; 3) establishing sensitive systems for surveillance of polio cases and poliovirus that rely on acute flaccid paralysis (AFP) reporting; and 4) carrying out "mopping-up" campaigns to eliminate the last foci of poliovirus transmission (2). This report describes progress toward polio eradication in Bangladesh during 1995-1997. The findings suggest that polio cases and wild poliovirus circulation are declining rapidly and that polio-eradication activities have effectively assisted in addressing other health priorities in Bangladesh. Routine Immunization Program The Expanded Program on Immunization (EPI) in Bangladesh was initiated in 1979. Intensification of EPI during 1985-1990 resulted in the establishment of a nationwide cold chain and approximately 120,000 health personnel trained according to WHO guidelines (3). From 1985 to 1990, reported coverage with OPV3 among children aged less than 12 months increased from 2% to virtually 100%. Although routine administrative reports indicate that coverage has been stable (87%-98%) since 1991 (Figure_1), annual independent surveys during 1992-1997 indicate that actual OPV3 coverage ranged from 60%-74% since 1991. National Immunization Days NIDs were conducted in Bangladesh in March and April 1995, April and May 1996, December 1996 and January 1997, and December 1997 and January 1998. The two most recent NIDs were conducted in coordination with countries near or bordering Bangladesh including Bhutan, India, Myanmar, and Nepal. In each year of NIDs, Bangladesh vaccinated greater than 90% of children aged less than 5 years with OPV. Independent surveys conducted after the first and third NIDs corroborated the high coverage reported by administrative method (Table_1). NIDs were successful despite frequent "hartals" (i.e., potentially violent political strikes in which all commercial and transportation facilities must remain closed). During a nationwide hartal on December 7, 1997 (the same day as round 1 of the fourth NIDs), to ensure high coverage, major polio-eradication partners, including WHO, Rotary International, and United Nations Chidren's Fund (UNICEF), jointly asked all persons in Bangladesh to recognize NIDs as National Days of Tranquility, and the government of Bangladesh mandated that the first round of NIDs be held for 2 consecutive days on December 7 and 8. The 99.1% coverage for round 1 was the highest ever reported in Bangladesh. Surveillance Data about reported cases of polio in Bangladesh have been available since 1988. Virologic testing of stool specimens for poliovirus began in 1992, and national AFP reporting began in 1996. In 1997, Bangladesh began implementation of a comprehensive plan for AFP and EPI Disease Surveillance** that includes development of facility- and community-based surveillance systems. Training has been provided to surveillance medical officers from 80 hospitals and to 1200 disease surveillance focal persons (DSFPs) and local surveillance officers (LSOs) at the municipality, city corporation, thana (i.e., a subdivision of a district), district, and division levels. DSFPs and LSOs conduct active weekly AFP surveillance at major health facilities and investigate any passively reported or actively identified AFP case. Incidence of Paralytic Polio The number of reported polio cases decreased from 520 in 1988 to approximately 230-290 cases per year during 1992-1994. From 1994 to 1995, the number of reported polio cases decreased from 289 to 207. In 1996, a total of 99 AFP cases were identified, of which 97 were confirmed as polio by the clinical classification method***, a 53% decrease compared with 1995 (Figure_1). As of January 7, 1998, Bangladesh has identified 234 cases of AFP with onset of paralysis in 1997, of which 167 (71.4%) have been confirmed, 12 (5.1%) discarded, and 55 (23.5%) are pending final classification as polio. Of the 167 polio cases occurring in 1997, 14 (8.4%) were confirmed by the presence of poliovirus in stool specimens, 34 (20.4%) by residual paralysis or weakness, two (1.2%) by death, and 117 (70.1%) by lack of follow-up examination. Among the 167 persons with polio in 1997, 26.0% were aged 5-14 years, an increase from 15.1% in 1996 and 7.7% in 1995. Case-patients resided in 49 of 64 districts in Bangladesh. As in previous years, cases occurred more commonly in early spring (March-April) and mid to late summer (July-September). Isolation of Poliovirus During 1997, the number of AFP case-patients for whom stool specimens were submitted substantially increased (Table_2). Intratypic differentiation (ITD) identified wild poliovirus from four poliovirus type 1 (P1) and two P3 isolates from 1994. ITD results from five P1 isolates from 1995 revealed two wild and three vaccine strains. In 1996, all of 15 P1 isolates tested were wild poliovirus, and a P2 isolate was vaccine-type. Three P1 isolates and a P3 isolate are pending ITD. In 1997, one of five P1 isolates was wild poliovirus; the remaining four were vaccine strains. Other isolates from 1997 are pending ITD (Table_2). During 1995-1997, wild poliovirus was isolated from cases in 13 of 64 districts in Bangladesh. Acute Flaccid Paralysis Surveillance In 1997, Bangladesh met three of the WHO-recommended AFP surveillance performance indicator criteria (1): 82% of AFP cases were investigated within 48 hours of notification (target 80%), 89% of stool specimens arrived at the polio laboratory within 72 hours of collection (target: 80%), and 85% of stool specimens had culture results available within 28 days of arrival at the laboratory (target: 80%). From 1996 to 1997, Bangladesh made substantial progress toward meeting three other WHO targets: the number of reported AFP cases increased from 99 to 234, and the nonpolio AFP rate increased from 0.004 to 0.03 per 100,000 children aged less than 15 years (target: 1 per 100,000); AFP cases with two stool specimens collected within 14 days of onset of paralysis increased from 23% to 44% (target: 80%); and AFP cases with 60-day follow-up examinations increased from 5% to 27% (Table_2). The percentage of AFP cases reported within 7 days of onset of paralysis (46%) remained stable. Reported by: Expanded Program on Immunization, Directorate General of Health Svcs, Ministry of Health and Family Welfare; Expanded Program on Immunization, World Health Organization, Dhaka, Bangladesh. World Health Organization Regional Office of South-East Asia, New Delhi, India. Global Program for Vaccines and Immunization, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Despite the substantial progress toward polio eradication in Bangladesh and other countries and regions of WHO (4-8), enhanced efforts are needed to ensure that polio is eradicated worldwide by 2000. NIDs in Bangladesh rapidly reduced the incidence of polio in 1995 and 1996 compared with previous years. More clinically confirmed polio cases were identified in 1997 than in 1996 because of a substantial increase in the number of AFP investigations without a 60-day follow-up examination. Ensuring 60-day follow-up examinations should decrease the number of confirmed polio cases and increase the nonpolio AFP rate. Bangladesh met WHO surveillance performance targets in 1997 for indicators related to case investigation rather than sensitivity and timeliness of AFP notification, reflecting the emphasis on training of hospital surveillance officers, DSFPs, and LSOs. Social mobilization of health facility staff and involvement of key informants for the immediate reporting of AFP cases from the community should improve both timeliness and sensitivity of AFP notification. Strengthened virologic surveillance in Bangladesh is needed to achieve polio eradication by 2000. However, the National Polio Laboratory has not yet achieved WHO accreditation. Intensive laboratory training and additional support for equipment should improve laboratory capability for sensitive and accurate poliovirus and NPEV identification. Polio-eradication activities in Bangladesh have addressed other health priorities. For example, NIDs have been associated with a dramatic increase in vitamin A coverage among children. Vitamin A coverage among children aged 1-5 years has doubled from 42%-50% before NIDs to 94%-100% during NIDs. In addition, NID orientation materials have included messages for identification and treatment of childhood pneumonia and diarrhea, and AFP and EPI disease surveillance have targeted AFP, neonatal tetanus, and outbreaks of measles for community reporting and all six EPI diseases for facility-based reporting. The achievements of routine EPI coverage in Bangladesh from 1985 to 1990 reinforced the value of disease prevention efforts. The success of NIDs with the involvement of hundreds of thousands of volunteers transformed EPI from a government program to a program in which community members participated in protecting their children from disease and malnutrition and ensuring the highest sustainable standard of health for children. Ongoing polio eradication priorities for Bangladesh include 1) improving routine coverage with OPV3; 2) enhancing AFP surveillance to meet WHO standards for surveillance performance; 3) achieving accreditation of the national laboratory as part of the WHO polio laboratory network; 4) continuing to conduct high-quality NIDs; and 5) planning for "mopping up" activities in areas with poliovirus circulation or at risk for poliovirus transmission. References
* Mass campaigns over a short period (days to weeks) during which two doses of OPV are administered to all children in the target group (usually aged 0-4 years) regardless of previous vaccination history, with an interval of 4-6 weeks between doses. ** External support provided by the government of Japan, Rotary International, U.S. Agency for International Development, UNICEF, WHO, and CDC. *** A confirmed case of polio is defined as AFP and at least one of the following: 1) a laboratory-confirmed wild poliovirus infection, 2) residual paralysis at 60 days, 3) death, or 4) no follow-up investigation at 60 days. Figure_1 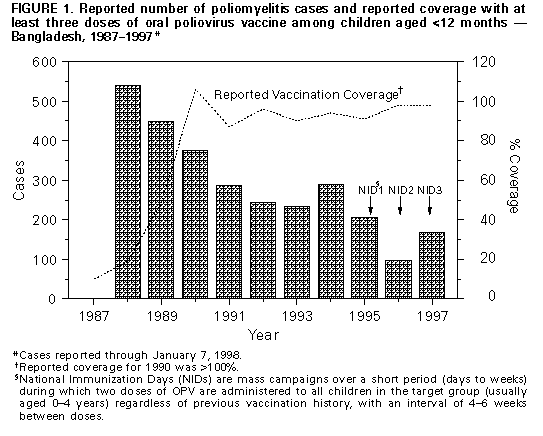 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Reported coverage with oral poliovirus vaccine (OPV) during each round of National Immunization Days
(NIDs)*, by date -- Bangladesh, 1995-1997
======================================================================================================================
OPV coverage
----------------------------------------------
First round Second round
-------------------- ---------------------
NID dates Estimated population aged <5 years Reported+ Survey Reported+ Survey
----------------------------------------------------------------------------------------------------------------------
March 1995; April 1995 18,937,880 83.9% 90% 92.3% 88%
April 1996; May 1996 19,318,535 92.8% NA& 99.9% NA
December 1996; January 1997 19,512,685 97.2% 95% 98.5% 92%
December 1997; January 1998 19,904,890 99.1% NA NA NA
----------------------------------------------------------------------------------------------------------------------
* Mass campaigns over a short period (days to weeks) during which two doses of OPV are administered to all
children in the target group (usually aged 0-4 years) regardless of previous vaccination history, with an interval
of 4-6 weeks between doses.
+ Reported by administrative method by the government of Bangladesh.
& Not available.
======================================================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Number and rate of reported poliomyelitis and acute flaccid paralysis (AFP) cases and stool specimen results, by year -- Bangladesh, 1992-1997
=====================================================================================================================================================================
Serotype
distribution of wild
poliovirus
isolated
-----------------------
Year* No. polio or AFP cases Overall AFP reporting Nonpolio AFP reporting No. polio or AFP cases P1 P2 P3
reported* rate+ rate+ with stool specimens
---------------------------------------------------------------------------------------------------------------------------------------------------------------------
1992 243 -- -- 11 -- -- --
1993 233 -- -- 65 -- -- --
1994 289 -- -- 68 4 0 2
1995 209 -- -- 37 2 0 0
1996 99 0.2 0.004 72 15 0 0
1997 234 0.5 0.032 226 1 0 0
---------------------------------------------------------------------------------------------------------------------------------------------------------------------
* Polio cases were reported from 1992 to 1996; AFP cases were reported beginning in 1996; year
refers to year of report for polio cases and year of paralysis onset for AFP cases.
+ Per 100,000 children aged <15 years.
& For 1997, sox poliovirus type 1 (P1) isolates, one P2 isolate, and t wo P3 isolates are pending intratypic differentiation.
=====================================================================================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|