 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- African Region, 1997In 1988, the World Health Assembly established the goal of eradicating poliomyelitis worldwide by 2000 (1). To achieve this goal, the World Health Organization (WHO) promotes the implementation of specific strategies (2,3). Eradicating polio from the African continent is one of the remaining major challenges to achieving global eradication by the target date. This report summarizes progress in the African Region of WHO in 1997 with the implementation of polio eradication strategies, and suggests that polio eradication by 2000 remains a feasible target. Reported routine coverage with three doses of oral poliovirus vaccine (OPV3) among children aged less than 1 year is low in the region overall but has increased from 47% in 1993 to 54% in 1996. In 1996, 12 countries reported that less than 50% of children were routinely vaccinated with OPV3. Of the largest and most populous countries (Angola, Democratic Republic of Congo {DR Congo}, Ethiopia, and Nigeria), only Ethiopia improved routine coverage (from 54% in 1995 to 67% in 1996), but coverage remained low in 1996 in Angola (42%), DR Congo (36%), and Nigeria (26%). All 24 countries of central and western Africa reported OPV3 coverage levels at less than 60% in 1996, except Algeria (77%), Benin (80%), The Gambia (97%), Senegal (80%), and Togo (82%). During 1997 and the first quarter of 1998, a total of 36 countries in the region conducted National Immunization Days (NIDs) * (Figure_1). These were the first NIDs for seven countries (Burundi, Guinea, Guinea-Bissau, Madagascar, Mali, Niger, and Senegal). Because of political instability, NIDs could not be conducted in Liberia, Republic of Congo, and Sierra Leone. Vaccination coverage was reported at greater than or equal to 80% for both rounds in all countries except Central African Republic (81% and 73%), Gabon (78% and 82%), Kenya (76% and 80%), Lesotho (67% and 65%), Mozambique (65% and 75%), Nigeria (72% and 91%), Rwanda (73%, first round results only), and South Africa (81% and 76%) (Table_1). DR Congo conducted Subnational Immunization Days (SNIDs) ** in 47 cities (25% of the total population); coverage was greater than 85% for both rounds. Nigeria conducted NIDs in 1996 and 1997, with reported coverage of 47% for the first and 75% for the second round in 1996, and 72% and 91% for first and second rounds, respectively, in 1997. In 1996, only five (16%) of 31 Nigerian states conducting NIDs reported coverage levels of greater than 80% in both rounds. In 1997, a total of 16 (43%) of 37 states implementing NIDs achieved greater than 80% coverage in both rounds. After 2 years of NIDs in Nigeria, 15 states did not reach coverage of greater than 80% in three of four rounds. In 1996, a total of 1949 polio cases were reported from the African region, with six countries accounting for 88% of cases: Nigeria (942), Ethiopia (264), DR Congo (219), Uganda (121), Chad (93), and Angola (81). In 1997, surveillance for acute flaccid paralysis (AFP) had been established in all but eight countries in the region (Burundi, Equatorial Guinea, Eritrea, Gabon, Liberia, Mali, Rwanda, and Sierra Leone). The rate of AFP reporting in each subregion (Western, Central, Southern, and Eastern) is low (average: less than 0.2 nonpolio AFP cases per 100,000 children aged less than 15 years). In two large countries that reported rates of nonpolio AFP of greater than 0.4 per 100,000 (Ghana and Uganda), the geographic distribution of AFP cases within the country was uneven, and the percentage of AFP cases with two specimens collected within 14 days of onset of paralysis remained below the level of greater than or equal to 80% recommended by WHO. In 1997, stool specimens collected from 73 persons with AFP in countries in east Africa (Kenya, Tanzania, Uganda, and Zambia) were negative for wild poliovirus, and no wild poliovirus was recovered in southern Africa. Wild poliovirus was isolated from 33 AFP cases from DR Congo and many countries in central and western Africa. Wild poliovirus also was isolated after the first NIDs in the Benin, Central African Republic, Chad, Cote d'Ivoire, and Nigeria. Partial genomic sequencing of several wild poliovirus isolates from countries neighboring DR Congo and Nigeria indicated that they are related to viruses found in DR Congo and Nigeria. Thirteen laboratories composing the African Regional Polio Laboratory Network -- three regional reference laboratories and 10 intercountry and national laboratories -- were fully functional in 1997. The network supports 31 countries in the region. Seven countries (Benin, Chad, DR Congo, Guinea, Guinea-Bissau, Ethiopia, and Mali) contributed specimens to network laboratories for the first time in 1997. Reported by: Expanded Program on Immunization, World Health Organization Regional Office for Africa, Harare, Zimbabwe. Global Program for Vaccines and Immunization, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine-Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Countries of the African Region made substantial progress toward polio eradication during 1996 and 1997 by 1) achieving high coverage during 2 years of conducting NIDs, 2) establishing AFP surveillance in many countries, and 3) creating a functional regional laboratory network. In addition, high-level political commitment and support for polio eradication in Africa achieved in 1996 was sustained in 1997. The two most important remaining reservoirs of wild poliovirus are Nigeria and DR Congo. In Nigeria, several states have not yet conducted one set of adequate double-round supplemental OPV vaccination during NIDs, and reported routine vaccination coverage with OPV3 was low during 1996. The first NIDs in DR Congo are scheduled to begin in August 1998. Surveillance data and genomic sequencing of viruses indicate that Nigeria and DR Congo are large remaining virus reservoirs that frequently export wild poliovirus to neighboring countries, making it more difficult for these countries to become polio free. AFP surveillance, although improving, remains at low levels. High-quality AFP surveillance is essential to assess the impact of polio eradication activities and, at later stages, to guide interventions aimed at the interruption of wild poliovirus transmission in the remaining virus reservoirs. Emphasis should be placed on active surveillance at the provincial level to improve the completeness and timeliness of detection, reporting, and investigation of AFP cases and the collection of appropriate stool specimens. Identifying personnel to conduct surveillance and ensuring transportation and operating expenses at the provincial level are important constraints. AFP surveillance in the African Region has already provided important epidemiologic information. Wild poliovirus was isolated widely even after the first NIDs in west and central African countries, indicating that wild poliovirus transmission had not yet been interrupted in those areas. In comparison with eastern and southern Africa, rapid success of polio eradication activities in west and central Africa is constrained further by lower levels of routine vaccination coverage in most countries. AFP surveillance represents the first surveillance system being implemented throughout the African Region that requires epidemiologic and virologic investigation of individual cases; its procedures are relatively complex and operationally demanding. Once fully established, AFP surveillance can facilitate surveillance, evaluation, and action for other diseases, including hemorrhagic fever, yellow fever, meningitis, epidemic dysentery, and other important and emerging diseases. Polio eradication in Africa is receiving increased external financial and technical support from Rotary International, WHO, United Nations Children's Fund (UNICEF), U.S. Agency for International Development (USAID), Basic Support for Institutionalizing Child Survival (BASICS) project, CDC, the government of Japan, the Canadian International Development Agency, vaccine manufacturers, and other partners. Polio eradication is achievable in the African Region by 2000 if the following constraints and potential obstacles are addressed:
References
Mass campaigns over a short period (days to weeks) during which two doses of OPV are administered to all children in the target age group (usually 0-4 years) regardless of previous vaccination history, with an interval of 4-6 weeks between doses. ** Focal mass campaigns in high-risk areas over a short period (days to weeks) in which two doses of OPV are administered to all children in the target age group, regardless of previous vaccination history, with an interval of 4-6 weeks between doses. Figure_1 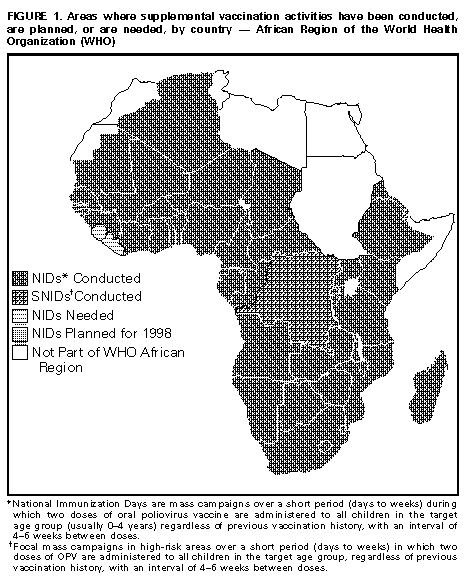 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Vaccination coverage with three doses of oral poliovirus vaccine (OPV3)
during 1996, and vaccination coverage during National Immunization Days (NIDs) *,
nonpolio acute flaccid paralysis (AFP) rates +, and reported number of polio cases,
during 1997, by countries with endemic polio -- African Region of the World Health
Organization
====================================================================================================
No.
1997 NID coverage Nonpolio confirmed
1996 OPV3 ------------------ AFP rate polio cases
Region/Country coverage Round 1 Round 2 1997 for 1997
----------------------------------------------------------------------------------------
Western
Algeria 77% 92% 92% 0.28 0
Benin 80% 100% 100% 0.01 2
Burkina Faso 48% 100% 100% 0.19 3
Cote d'Ivoire 55% 100% NR& 0.12 3
Chad 20% 91% 99% 0.14 2
Gambia @ 97% -- -- 0.25 0
Ghana 52% 98% NR 0.42 4
Guinea 48% 100% 100% 0.10 0
Guinea-Bissau 54% NR NR 0.20 0
Liberia ** 45% -- -- ++ NR
Mali 52% 95% 100% ++ NR
Mauritania 50% 90% 93% 0.60 0
Niger 23% 88% 95% 0.14 6
Nigeria 26% 72% 91% 0 4
Senegal 80% 97% 100% 0.19 2
Sierra Leone ** 65% -- -- ++ NR
Togo 82% 99% 100% 0.13 1
Central
Angola 42% 83% 90% 0.24 7
Cameroon 46% 91% 100% 0.17 11
Central African Republic 53% 81% 73% 0.19 8
Congo ** 50% -- -- 0 NR
Democratic Republic
of Congo 36% 95% && 85% && 0.07 6
Equatorial Guinea 64% 89% 100% ++ NR
Gabon 41% 78% 82% ++ NR
Southern
Botswana 81% 97%&& 81% 0.57 0
Lesotho 58% 67% 65% 0.11 0
Madagascar 73% 100% 100% 0.19 0
Malawi 82% 96% 100% 0.20 0
Mozambique 60% 65% 75% 0.05 0
Namibia 71% 100% 95% 0.71 2
South Africa 73% 81% 76% 0.32 0
Swaziland 71% NR NR 0.50 0
Zimbabwe 76% 96% 96% 0.82 0
Eastern
Burundi 63% NR NR ++ NR
Eritrea 46% 82% 84% ++ 0
Ethiopia 67% 88% NR 0.05 NR
Kenya 43% 76% 80% 0.11 0
Rwanda 99% 73% NR ++ 0
Tanzania 82% 95% 98% 0.11 NR
Uganda 79% 92% 94% 0.41 3
Zambia 83% 96% 87% 0.15 0
----------------------------------------------------------------------------------------
* Mass campaigns over a short period (days to weeks) during which two doses of oral
poliovirus vaccine are administered to all children in the target age group (usually 0-4 years)
regardless of previous vaccination history, with an interval of 4- 6 weeks between doses.
+ Per 100, 000 children aged <15 years.
& Not reported.
@ NIDs not needed.
** NIDs not conducted because of political instability.
++ AFP surveillance system not yet established.
&& Conducted Subnational Immunization Days, which are focal mass campaigns in high-risk
areas over a short period (days to weeks) in which two doses of OPV are administered to
all children in the target age group, regardless of previous vaccination history, with an
interval of 4-6 weeks between doses.
====================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|