 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity -- United States, 1998-99 SeasonThis report summarizes influenza activity in the United States from October 4, 1998, through February 27, 1999. It also presents results of an investigation of an influenza outbreak among staff and residents at one long-term-care facility (LTCF), and estimates the 1998-99 influenza vaccine effectiveness against the circulating influenza A(H3N2) viruses at that facility. Based on influenza surveillance data, influenza activity in the United States began to increase in mid-January 1999 and remained elevated in most regions of the country through the week ending February 27. The percentage of patient visits to approximately 350 sentinel physicians for influenza-like illness (ILI) increased from baseline levels of 0-3% during the week ending January 23 and has remained elevated for 6 consecutive weeks. For the week ending February 27, 4% of patient visits were for ILI. Visits for ILI were above baseline levels in all influenza surveillance regions for the week ending February 27 except the mid-Atlantic and east south central regions, which had levels of 1% and 3%, respectively. Since the week ending January 23, at least 25 states have reported either widespread or regional activity each week (Figure_1). The highest number of states reporting either widespread or regional activity during any 1 week was 43 during the week ending February 13. State and territorial epidemiologists in 41 states and the District of Columbia reported either widespread or regional influenza activity * for the week ending February 27. The percentage of deaths attributed to pneumonia and influenza (P&I) among 122 U.S. cities was 8.1% for the week ending February 27, which is above the epidemic threshold ** of 7.5%. Mortality from P&I exceeded the epidemic threshold for 3 consecutive weeks beginning the week ending February 13. From October 4, 1998 through February 27, 1999, the World Health Organization and the National Respiratory Enteric Virus Surveillance System collaborating laboratories in the United States reported detection of influenza in 6529 (12%) of 52,355 clinical specimens submitted for respiratory virus testing. Of the influenza-positive specimens, 5170 (79%) were type A and 1359 (21%) were type B. Of the 5170 influenza A isolates, 1275 (25%) were H3N2 viruses, 12 (0.2%) were H1N1 viruses, and 3883 (75%) were not subtyped. In the west north central, east north central, and east south central regions, 35%-46% of the influenza isolates were type B. Of 169 influenza A(H3N2) isolates collected during October 4, 1998-February 27, 1999, that were antigenically characterized at CDC, all were characterized as A/Sydney/5/97-like viruses, the H3N2 virus strain contained in the 1998-99 influenza vaccine. Two influenza A(H1N1) isolates were characterized as A/Bayern/7/95-like viruses, antigenically distinct from A/Beijing/262/95, the 1998-99 H1N1 vaccine strain; however, A/Beijing/262/95 produced high titers of antibodies that cross-react with A/Bayern/7/95. All 51 influenza type B isolates were antigenically similar to B/Beijing/184/93, the recommended type B vaccine strain. Long-Term-Care Facility Outbreak The California Department of Health Services (CDHS) requires that all LTCFs report respiratory illness outbreaks to the state or local health department. As of February 27, CDHS had received five reports of culture-confirmed influenza outbreaks among the approximately 1200 LTCFs in the state. Following is a result of an investigation of one of these outbreaks. On December 31, 1998, a LTCF notified the Santa Clara County Public Health Department of an ILI outbreak among residents of two units in one of the facility's four buildings. Nasopharyngeal swab specimens from eight of 10 ill residents were positive for influenza A by direct fluorescent antibody testing. The outbreak investigation included active surveillance for ILI (temperature greater than or equal to 100 F {greater than or equal to 38 C} and cough or sore throat or rhinitis), viral culture of nasopharyngeal swab samples collected from selected ill residents and staff, and collection of vaccination and illness histories from residents and staff in the two affected units. Vaccine effectiveness against ILI was calculated as 1 minus relative risk. Residents in this facility are assigned to different buildings according to the level of care required. The most debilitated residents, most of whom are bedridden and require complete care, reside in Building 1. During the fall, residents in all four buildings (n=524) received influenza vaccination, except residents with medical contraindications. Of the 1200 staff members offered vaccine, approximately 200 (17%) were vaccinated at the facility, and some may have been vaccinated by outside providers. The first cases of ILI occurred during December 21-December 28, 1998, among five unvaccinated nurses who worked in two adjacent units in Building
Vaccine effectiveness against ILI was 72% (95% CI: -1.3-92.4) among the 47 staff members. Vaccine effectiveness was not estimated for residents because of the small number of unvaccinated persons. Four influenza A(H3N2) isolates obtained from ill residents were antigenically characterized as A/Sydney/5/97-like viruses. Outbreak-control measures included cohorting ill residents and initiating droplet precautions (1) and administering amantadine for prophylaxis of non-ill residents and treatment of ill residents. Unvaccinated staff were offered amantadine prophylaxis and influenza vaccine. Ill staff were discouraged from coming to work, and ill visitors were asked to postpone their visits. Reported by: SH Cody, MD, AF Bolding, M Fenstersheib, MD, GS Olivas, PhD, Santa Clara County Public Health Dept; C O'Malley, PhD, N Smith, MD, Immunization Br, M Hendry, DSc, Respiratory, AIDS, and Support Section, Viral and Rickettsial Disease Laboratory; S Waterman, MD, State Epidemiologist, California Dept of Health Svcs. Participating state and territorial epidemiologists and state public health laboratory directors. National Respiratory Enteric Virus Surveillance System collaborating laboratories. World Health Organization collaborating laboratories. Sentinel Physicians Influenza Surveillance System. WHO Collaborating Center for Reference and Research on Influenza, Influenza Br, and Respiratory Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: Almost all states have reported either regional or widespread influenza activity this influenza season. Although only 21% of influenza isolates have been type B, influenza B viruses have been detected in all influenza surveillance regions. Influenza A/Sydney/5/97 (H3N2)-like virus appears to be the predominant strain so far this influenza season. The influenza A outbreak described in this report illustrates several points. First, influenza outbreaks can occur among highly vaccinated LTCF populations even in years when the vaccine is well matched to circulating virus strains (2,3); LTCFs should conduct surveillance to identify clusters of respiratory illness and should alert state or local health departments when clusters are identified. Second, early detection of influenza outbreaks and timely initiation of control measures, such as cohorting of ill residents, use of droplet precautions, and use of antiviral medications (amantadine or rimantadine) for prophylaxis or treatment of persons at high risk for influenza A-related complications, can limit the spread of disease (1,4). Amantadine and rimantadine are 70%-90% effective in preventing influenza A infections and can reduce severity and duration of symptoms from influenza A when administered within 48 hours of onset; however, these medications are not effective against influenza type B viruses (5). Chronic-care facilities should know which laboratories in their area perform rapid influenza A testing and should develop a plan to rapidly detect influenza A outbreaks and to administer antiviral medications if influenza is detected (4-7). Third, health-care workers can act as a vehicle for introducing influenza illness into LTCFs (3,7). Because influenza infections can be severe in debilitated populations and because vaccine effectiveness is lower among LTCF residents (30%-40%) than in healthy adults (70%-90%), the Advisory Committee on Immunization Practices recommends that health-care workers and others caring for high-risk persons receive influenza vaccine annually (2,3,5,7). Health-care workers and family members should be educated about the potentially serious consequences of influenza illness for high-risk persons and the need to limit contact with these persons. When health-care workers and family members are ill, they should avoid contact with high-risk persons. Influenza surveillance data collected by CDC are updated
weekly
throughout the influenza season. Summaries are available through
CDC;
telephone (888) 232-3228, or fax (888) 232-3299 (request document
number
361100). Surveillance information also is available on the
World-Wide Web
at References
Levels of activity are 1) no activity; 2) sporadic -- sporadically occurring ILI or culture-confirmed influenza with no outbreaks detected; 3) regional -- outbreaks of ILI or culture-confirmed influenza in counties with a combined population of less than 50% of the state's total population; and 4) widespread -- outbreaks of ILI or culture-confirmed influenza in counties with a combined population of greater than or equal to 50% of the state's total population. ** The epidemic threshold is 1.645 standard deviations above the seasonal baseline. The expected seasonal baseline is projected using a robust regression procedure in which a periodic regression model is applied to observed percentages of deaths from P&I since 1983. Figure_1 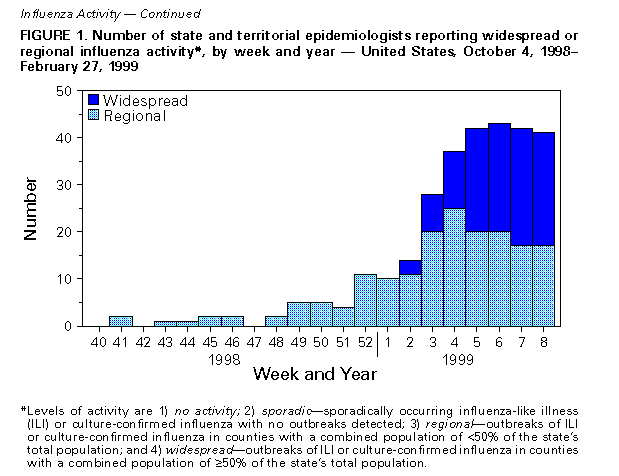 Return to top. Figure_2 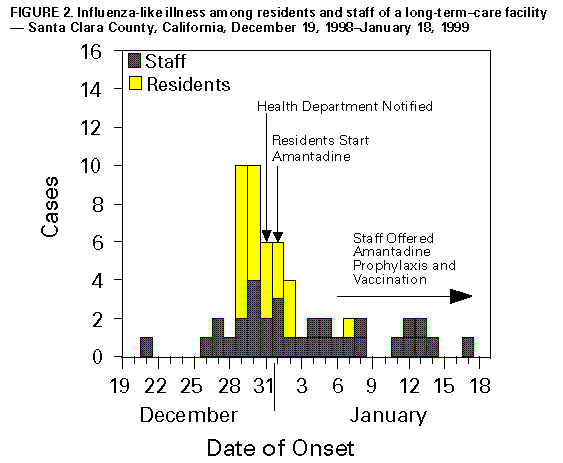 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 03/11/99 |
|||||||||
This page last reviewed 5/2/01
|