 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- Nigeria, 1996-1998In 1988, the World Health Assembly resolved to eradicate poliomyelitis globally by 2000 (1). In the African Region of the World Health Organization (WHO), eradication efforts were accelerated following supporting resolutions by WHO's Regional Committee for Africa in 1995 (2,3) and the Organization of African Unity in 1996 (4). Nigeria, the most populous country in Africa and part of a densely populated West African area extending from Nigeria to Cote D'Ivoire, is critically important to the global polio eradication initiative. This report summarizes 1) the success of National Immunization Days (NIDs) *; 2) the establishment of acute flaccid paralysis (AFP) surveillance; and 3) accelerated efforts to meet the 2000 target, including mopping-up ** planned for later in 1999. Routine Vaccination Coverage During 1994-1997, reported routine vaccination coverage with three doses of oral poliovirus vaccine (OPV) among infants aged less than 1 year nationwide remained at low levels: 34% in 1994, 29% in 1995, 21% in 1996, and 25% in 1997. These suboptimal coverage rates varied substantially by state within Nigeria. National Immunization Days In 1996, Nigeria initiated NIDs, and reported nationwide OPV coverage was 47% after the first round in November and 75% after the second round in December (5). In 1997, nationwide coverage was 76% following the first round of NIDs and 94% after the second round (6). Nationwide coverage of the third NIDs was 100% in the first round in November 1998 and 108% in the second round in December 1998 ***. In 1998, reported coverage during round one ranged from 63% in Imo State to 147% in Katsina State. Acute Flaccid Paralysis Surveillance AFP surveillance was initiated in December 1996 with a pilot project in Lagos. The number of AFP cases identified increased from eight in 1997 to 525 in 1998. As of April 1999, 327 AFP cases have been confirmed as polio (40 by wild poliovirus isolation and 287 by clinical case classification criteria {i.e., residual paralysis of 60 days or no follow-up because the person had died or could not be found}). The total AFP rate was 1.1 per 100,000 children aged less than 15 years, and the nonpolio AFP rate was 0.4 (target: one nonpolio AFP case per 100,000 children aged less than 15 years). The number of AFP cases for which stool specimens were available increased from 10 cases in January to 112 cases in September 1998 (Figure_1). The rapid increase in the number of AFP cases was associated with funding for personnel and transportation to conduct active surveillance at the state and local government (district) level. The number of AFP cases declined substantially during October-December 1998, probably as a result of both a seasonal decline and problems with release of funds for surveillance. In 1998, AFP cases for which stool specimens were available were identified in 36 of 37 states (Figure_2). Of the 37 states, 24 had an AFP rate of greater than or equal to 0.5 cases per 100,000 children aged less than 15 years. Among 517 AFP case-patients with specimens in 1998, 378 (73%) had at least one stool specimen collected less than 30 days from paralysis onset, and 43% had at least one specimen collected within 14 days of paralysis onset. Eighty-five percent of AFP case-patients had two specimens collected, and 37% had two specimens collected within 14 days of paralysis onset. Stool specimen isolation results were available for 269 (52%) of 517 AFP cases. Results were not available for 19% of AFP cases with specimens with onset in June 1998, 58% with onset in August 1998, and 100% with onset in October 1998. Of the 269 AFP cases with stool specimens with results, wild poliovirus was isolated in 40 (34 had type 1; one, type 2; and five, type 3) (Figure_2). Mopping-Up Two house-to-house, mopping-up OPV vaccination rounds are planned for 15 of 37 states in April and May 1999, targeting 13 million children aged less than 5 years (representing 52% of the total population aged less than 5 years). States that will conduct mopping-up meet one or more of the following criteria: coverage less than 80% during two or more rounds in the 1997 and 1998 NIDs, wild poliovirus isolated in 1998, AFP rate less than 0.5 cases per 100,000 children aged less than 15 years in 1998, and densely populated areas with poor surveillance and/or cities with a population greater than 750,000 persons. Reported by: Expanded Program on Immunization, Ministry of Health, Abuja; World Health Organization, Lagos, Nigeria. Regional Office for Africa, World Health Organization, Harare, Zimbabwe. Vaccines and Biologicals, World Health Organization, Geneva. Respiratory and Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial NoteEditorial Note: The findings in this report indicate that wild poliovirus transmission remains widespread in Nigeria. Although the quality of NIDs has improved each year, NID coverage has not been high enough to eradicate the virus. Interruption of poliovirus transmission by 2000 will require additional supplemental vaccination rounds. Mopping-up rounds in April and May 1999 will be among the first large-scale, house-to-house vaccination activities in Africa. To ensure high-quality NIDs in the future, additional strategies (e.g., extensive use of house-to-house vaccination and dose monitoring the number of unvaccinated children) may be needed. Of the 40 AFP cases with wild poliovirus, 24 were in states that are not targeted for mopping-up. Results of pending stool specimens and AFP surveillance from January to May 1999 will be critical in determining whether additional states need to be targeted for mopping-up activities. AFP surveillance needs to be maintained at high levels. The rapid decline of AFP surveillance during October-December 1998 resulted, in part, from diversion of active surveillance personnel for supplemental vaccination activities. Adequate administrative methods to deliver funding must be developed and additional field staff may be needed to avoid this problem. Solutions are needed for the delay in stool specimen processing. Because 48% of AFP cases with stool specimens are pending laboratory processing, important information is missing that forms the basis for directing vaccination efforts. Several activities have been initiated to resolve the backlog of unprocessed stool specimens, including adding staff at the Ibadan laboratory, forwarding 119 specimens to the Ghana laboratory, and opening a second national laboratory in Nigeria that is nearly ready to accept AFP stool specimens. Nigeria and West Africa are among the few remaining reservoirs of wild poliovirus transmission in the world (7,8). Interruption of wild poliovirus transmission will require 1) successful mopping-up in 15 states during April and May 1999; 2) high quality mopping-up in additional states guided by surveillance before the start of NIDs in November 1999; 3) house-to-house vaccination during the next two NIDs to assure high coverage; 4) statewide house-to-house mopping-up in any state with wild poliovirus transmission during 2000; and 5) maintenance and further strengthening of AFP surveillance. Nigeria's polio eradication efforts are supported by WHO, United Nations Children's Fund (UNICEF), Rotary International, U.S. Agency for International Development, and CDC. References
Nationwide mass campaigns over a short period (days to weeks), in which two doses of oral poliovirus vaccine are administered to all children in the target age group (usually aged less than 5 years), regardless of vaccination history, with an interval of 4-6 weeks between doses. ** Focal mass campaigns in high-risk areas during a short period (days to weeks) in which two doses of oral poliovirus vaccine are administered during house-to-house visits to all children in the target age groups, regardless of vaccination history, with an interval of 4-6 weeks between doses. *** Reported coverage rates greater than 100% may result from inaccurate numerator and denominator data or vaccination of children outside the target age group (i.e., aged greater than 5 years). Figure_1 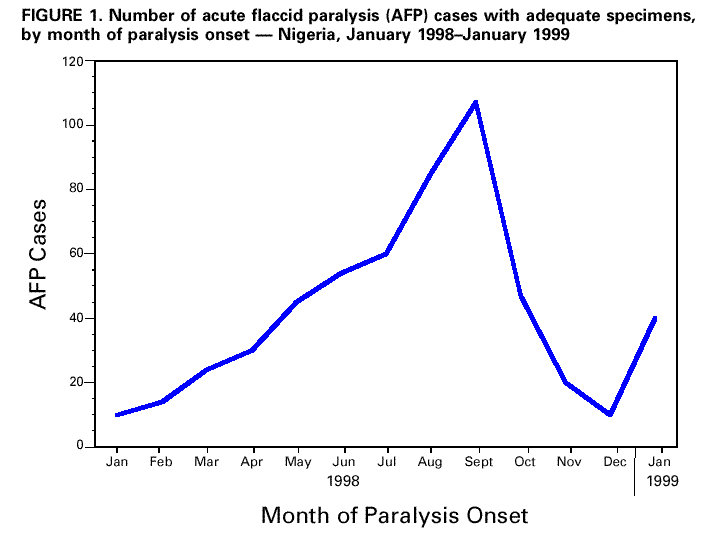 "> ">Return to top. Figure_2 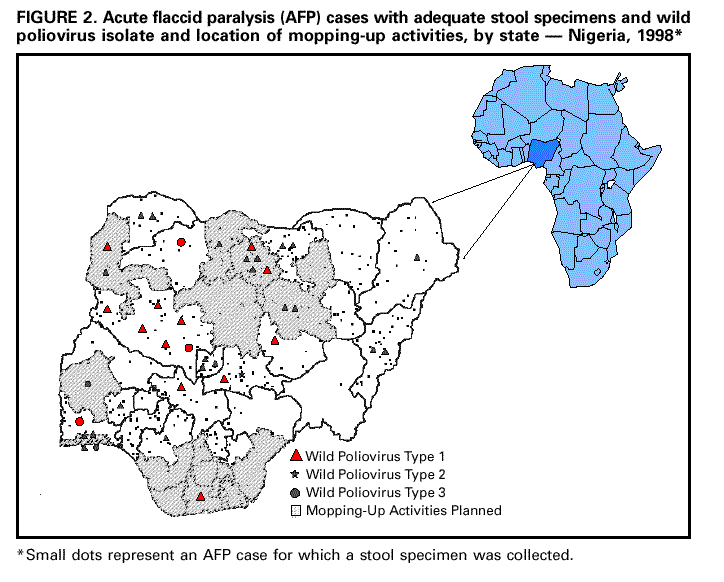 "> ">Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 04/22/99 |
|||||||||
This page last reviewed 5/2/01
|