 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- Chad, 1996-1999In 1988, the World Health Organization (WHO) resolved to eradicate poliomyelitis globally by December 31, 2000 (1). Polio eradication activities have been conducted in WHO's African Region (AFR) since 1996, and recently have been accelerated (2). In 1997, central African countries began conducting National Immunization Days (NIDs)* and established surveillance systems for acute flaccid paralysis (AFP). This report summarizes progress toward polio eradication and the establishment of AFP surveillance in Chad. Routine Vaccination Chad (population: 6.4 million) is a republic in central sub-Saharan Africa and is the fifth largest African country in area. After three decades of civil war, the damage to Chad's infrastructure affected the delivery of health-care services, including vaccination coverage. Most health-care providers practice in the capital, N'djamena, and few trained personnel are stationed in periurban and rural areas. Since 1990, reported routine infant vaccination coverage (e.g., three doses of oral poliovirus vaccine [OPV]) has been 10%-25%. This percentage is consistent with the continued reporting of clinically and virologically confirmed polio cases from the most populated areas of southern Chad (Figure 1). Supplementary Vaccination Activities Chad implemented NIDs with OPV for the first time during February-March 1997, followed by two more rounds during February-March 1998 and November-December 1998. Approximately 800,000 (90%) children aged less than 5 years were vaccinated during each round. Vaccine vial monitors (VVMs)** were used to ensure that potent vaccine was administered at each vaccination site. Two rounds of intensified, door-to-door NIDs were conducted in December 1999 and January 2000. Coverage data are not yet available. AFP Surveillance During 1995-1997, Chad collected information about clinically confirmed cases of paralytic polio through a passive surveillance system; 402, 331, and 326 clinically diagnosed polio cases in 1995, 1996, and 1997, respectively, were reported; however, reporting accuracy and completeness during this period are questionable because no standard case definition was used and few of the cases were investigated further (3). In 1997, AFP and wild poliovirus surveillance began to include clinical and virologic case investigations; stool specimens were collected from four AFP cases. Despite shipment delays, the regional reference laboratory in Bangui confirmed wild poliovirus type 1 in one specimen. AFP case reporting and stool specimen collection increased in 1998; 14 AFP cases were identified and investigated from January to October 1998, confirming wild poliovirus type 1 in four cases (Table 1). Surveillance activities decreased in late 1998; no cases were reported from October 1998 to April 1999. In May 1999, the Ministry of Health (MOH) established the national service of integrated active surveillance to monitor AFP, measles, malaria, yellow fever, meningitis, and cholera. Five national surveillance officers began to train health-care personnel on active AFP surveillance in all provinces, and the first international team of three WHO/CDC Stop Transmission of Polio epidemiologists were sent to Chad. In 1999, 182 AFP cases were reported; two stool specimens were collected from 133 of these cases. Forty-nine AFP cases were identified through retrospective record review, and these cases were subsequently confirmed as polio using the clinical classification system. As of December 1999, the Bangui regional reference laboratory reported final virus isolation and intratypic differentiation results for 85 of the 133 AFP cases for which two specimens were collected. Wild poliovirus was confirmed in 33 of 85 cases. Results have not been reported for 48 of the 133 cases for which two specimens were collected. Two of the main indicators used to monitor AFP surveillance are the reported nonpolio AFP rate (4), which is used to assess the sensitivity of detection and accuracy of reporting suspected cases (target: a rate of greater than 1 nonpolio AFP case per 100,000 children aged less than 15 years annually), and the proportion of AFP cases from which two specimens have been collected within 2 weeks of paralysis onset (target: two adequate stool specimens from greater than 80% of AFP cases). In Chad in 1999, the nonpolio AFP rate was 1.49***. Two stool specimens within 2 weeks of paralysis onset were collected from 46% of reported AFP cases; specimen arrival at the laboratory within 3 days occurred in 11% of the cases, and 11% of the persons with cases received clinical follow-up examinations. Reported by: Integrated Surveillance Unit and Expanded Program of Immunization, Ministry of Health, N'djamena, Republic of Chad; World Health Organization Country Office, N'djamena, Republic of Chad. World Health Organization Regional Office for Africa, Harare, Zimbabwe. Vaccines and Other Biologicals Dept, World Health Organization, Geneva, Switzerland. Robert Koch Institute, Berlin, Germany. Epidemiology and Surveillance Div and Vaccine Preventable Disease Eradication Div, National Immunization Program; and an EIS Officer, CDC. Editorial Note:Chad connects western and central Africa where polio is endemic. Three decades of civil war have left Chad with a damaged health infrastructure, severe financial problems, and limited human resources. These factors and a large mobile population (e.g., nomads, migrant workers, and refugees) have led to low routine coverage with three doses of OPV and continued widespread transmission of wild poliovirus. Chad's MOH is increasingly successful in implementing WHO's recommendations for supplemental OPV vaccination and intensified surveillance. Enhanced AFP and wild poliovirus surveillance has demonstrated that Chad is still a substantial reservoir for poliovirus transmission in Africa. Critical challenges to surveillance are lack of transportation and communication, inaccessibility of some regions during the rainy season (June to October), technical problems in conserving and transporting stool specimens, and population movements that make follow-up difficult. Improved surveillance will depend on better coordination among all levels of government and local nongovernmental organizations, and cooperation across international borders. MOH priorities for 2000 are to implement high quality NIDs, particularly in the populated areas of southern Chad and among nomadic groups, and to maintain and improve the quality of AFP surveillance. Progress in these areas should enable Chad to reach the polio eradication goal. References
* Mass campaigns over a short period (days to weeks) in which two doses of oral poliovirus vaccine are administered to all children, usually aged less than 5 years, regardless of vaccination history, with an interval of 4-6 weeks between doses. ** A heat-sensitive label that changes color if vaccine has been exposed to heat, which degrades the vial contents. *** Nonpolio AFP rate is calculated using the 52 cases discarded as nonpolio through negative laboratory results. Table 1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 1. Reported cases of acute flaccid paralysis (AFP), confirmed poliomyelitis, confirmed wild virus, and nonpolio AFP rate -- Chad, 1995-1999
* Calculated as number of AFP cases not caused by polio per 100,000 population aged <15 years. Source: World Health Organization (WHO) African Region Expanded Program of Immunization Plan of Action 1999, Ministry of Health, Ndjamena, Chad, and WHO polio eradication update: available on the World-Wide Web at http://www-nt.who.int/vaccines/polio/case.asp. References to sites of non-CDC organizations on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages at these sites. Return to top. Figure 1 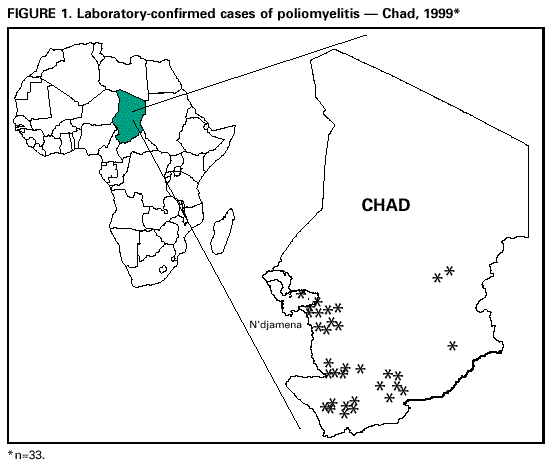 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/26/2000 |
||||||||||||||||||||||||||||||||
This page last reviewed 5/2/01
|