 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Influenza B Virus Outbreak on a Cruise Ship --- Northern Europe, 2000During June 23--July 5, 2000, an outbreak of respiratory illnesses occurred on the MS Rotterdam (Holland America Line & Windstar Cruises) during a 12-day Baltic cruise from the United Kingdom to Germany via Russia. The ship carried 1311 passengers, primarily from the United States, and 506 crew members from many countries. Although results of rapid viral testing for influenza A and B viruses were negative, immunofluorescence staining and viral culture results implicated influenza B virus infection as the cause of the outbreak. This report summarizes the findings of the outbreak investigation conducted by the ship's medical department and describes the measures taken to control the outbreak. Travelers at high risk for complications of influenza who were not vaccinated with influenza vaccine during the preceding fall or winter should consider receiving influenza vaccine before travel with large tourist groups at any time of year or to certain regions of the world. On June 26, nine crew members presented to the ship's infirmary with cough, sore throat, and fever >100.0 F (>37.8 C ). All had developed symptoms during the preceding 24 hours. Oropharyngeal specimens from two crew members were tested by a commercial rapid influenza diagnostic test designed to detect both influenza A and B viruses but not to distinguish between them. Although test results were negative, three crew members with high fevers were started on rimantadine therapy for clinically suspected influenza A infection. To characterize and control the suspected outbreak among crew members, ship's medical staff implemented a respiratory illness protocol that included surveillance for cases of respiratory illness. A case of acute respiratory illness (ARI) was defined as cough or sore throat. Influenza-like illness (ILI), a subset of ARI cases, was defined as ARI with fever >100.0 F (>37.8 C) or self-reported feverishness. Active surveillance was initiated among crew members. Supervisors on each work shift observed and asked crew members about symptoms of influenza and required any crew member with symptoms to report to the ship's infirmary for evaluation. Crew members with confirmed ILI were relieved of duty and placed in cabin isolation either alone or with other ill crew members. Passive surveillance was initiated among passengers and identified any passenger who presented to the ship's infirmary with respiratory illness. A commercial rapid influenza diagnostic test, designed to detect both influenza A and B viruses but not to distinguish between them, was used selectively to assist in diagnosis. Medical and demographic information, including country of residence, cabin number, and crew duties (if applicable), was collected from ill patients. By June 29, 38 crew members and 26 passengers had been seen in the infirmary for ARI; of these, 32 (84%) crew members and 11 (42%) passengers had ILI. Eight crew members were tested by rapid influenza diagnostic testing; all had negative results. Because the etiology of crew respiratory illnesses remained uncertain, four symptomatic crew members disembarked in Stockholm, Sweden, for medical evaluation that included testing of nasopharyngeal specimens by immunofluorescence staining and viral culture. Two of four nasopharyngeal specimens tested positive for influenza B virus by immunofluorescence staining; one of the two specimens also was positive by culture. Neither of the two crew members diagnosed with influenza B virus infection had been tested using the rapid influenza diagnostic test. On the basis of immunofluorescence results, crew members on rimantadine therapy, which is effective only against influenza A infection, were advised to discontinue their medication. Oseltamivir, an antiviral agent that is effective against both influenza A and B infection, was sent to the ship for treatment of ill crew members and passengers. A total of 64 (13%) crew members and 54 (4%) passengers were identified with ARI during the cruise. Of 63 crew members and 54 passengers with ARI for whom clinical information was known, 45 (71%) and 25 (46%), respectively, also had ILI (Figure 1). The median age of ill crew members was 32 years (range: 21--56 years) and of passengers, 68 years (range: 7--85 years). By cross-referencing crew duties, cabin locations of ill crew members and passengers, and dates of illness, medical staff identified the potential index case-patient as a 78-year-old U.S. passenger who boarded the ship ill with unconfirmed ILI after visiting London. She remained in her cabin except for occasional meals and did not seek medical attention until the fifth day of the cruise (June 28). Two of the 13 crew members with ILI, who were seen in the infirmary on June 25 and 26, were her cabin and dining room stewards. Both had worked, socialized, or shared cabins with other crew members who became ill. Surveillance among passengers and crew members was continued during the subsequent cruise and showed a decrease in the number of ARI and ILI cases. Reported by: SE Christensen, RC Wolfmeyer, SM Suver, CD Hill, MD, Holland America Line & Windstar Cruises, Seattle, Washington. SFF Britton, MD, Karolinska Institute, Stockholm, Sweden. Influenza Br, Div of Viral and Rickettsial Diseases; Surveillance and Epidemiology Br, Div of Quarantine, National Center for Infectious Diseases, CDC. Editorial Note:The findings of this investigation implicated influenza B virus as the cause of a respiratory illness outbreak onboard a cruise ship. Although the results of rapid viral testing for influenza A and B viruses were negative, influenza B infection was confirmed by viral culture and immunofluorescence antibody testing in two crew members. Although these tests were not performed on passengers, epidemiologic evidence suggested that respiratory illness cases among crew members and passengers were related and that an ill passenger might have transmitted infection to crew members. Rapid viral diagnostic testing for influenza can be useful for patient management and influenza outbreak control. However, these tests are not as accurate in detecting influenza infection as viral culture (1). If an influenza outbreak is suspected, nasopharyngeal specimens should be collected simultaneously for rapid viral tests and viral isolation. Viral isolation is essential for identifying new or unusual strains of influenza and for selecting influenza vaccine strains. Influenza A outbreaks have been reported on cruise ships sailing in the Northern Hemisphere during the summer, but influenza B outbreaks have not been documented (2--7). Early suspicion of a potential influenza outbreak among crew members and rapid implementation of a respiratory illness control protocol probably limited the size of the outbreak. Key elements of the protocol included 1) implementation of active and passive surveillance using standard case definitions; 2) use of targeted rapid influenza diagnostic testing and viral cultures to confirm cases of influenza virus infection; 3) isolation of all crew members meeting the ILI case definition or those with confirmed influenza; 4) use of antiviral agents for treatment and, if indicated, for prophylaxis; and 5) monitoring of intervention results (8). Because influenza viruses usually are spread by droplets and aerosols produced by an infected person who is coughing or sneezing, isolation can limit the spread of infection in semienclosed environments such as cruise ships (2). Although the number of days crew members with ILI were isolated from noninfected crew members and passengers was not reported, isolation measures ideally should have covered the first 5 days of illness, a period based on the duration of influenza virus shedding in adults (8). Summertime influenza outbreaks among passengers and crew members on cruise ships suggest that traveling in large groups can pose a risk for exposure to influenza viruses, even when the group is traveling in regions where influenza is not in seasonal circulation. Both passengers and crew members can serve as potential reservoirs of influenza infection. Travelers at high risk for complications of influenza (e.g., persons aged >50 years, immunocompromised persons, and persons with chronic disorders of the pulmonary or cardiovascular systems) who were not vaccinated with influenza vaccine during the preceding fall or winter should consider receiving influenza vaccine before travel 1) with large organized tourist groups at any time of year; 2) to the tropics; or 3) to the Southern Hemisphere from April through September (the time of increased influenza activity in that hemisphere) (9). Cruise lines should attempt to achieve at least an 80% vaccination rate among crew members on each ship each year (8). References
Figure 1 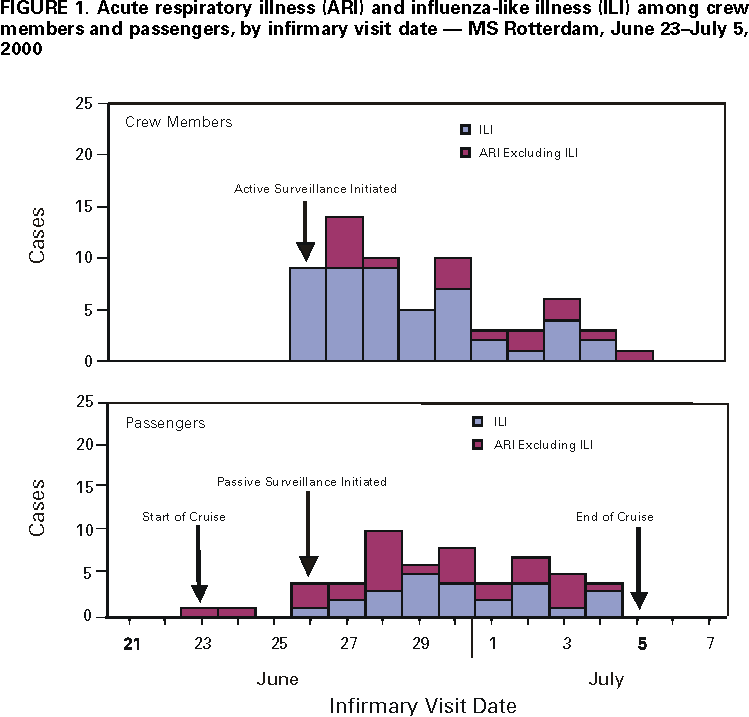 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/1/2001 |
|||||||||
This page last reviewed 5/2/01
|