 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Evaluation of a Regional Pilot Program to Prevent Mother-Infant HIV Transmission --- Thailand, 1998--2000Worldwide, approximately 2.2 million women and 600,000 infants are infected with human immunodeficiency virus (HIV) each year (1). Extended zidovudine prophylaxis and other antiretroviral and obstetric interventions and the avoidance of breastfeeding have reduced dramatically mother-infant HIV transmission in countries with adequate health-care resources (2,3). However, in developing countries, where the impact of HIV is greatest, implementation has been limited by the complexity and expense of these interventions (4). In Thailand, where approximately 15,000 infants are born to HIV-infected women each year, the Ministry of Public Health (MOPH) has collaborated with other organizations to identify simpler and more cost-effective interventions to reduce mother-infant HIV transmission. In 1998, a placebo-controlled clinical trial in Thailand using a simplified zidovudine regimen from 36 weeks' gestation until delivery reduced the risk for mother-infant transmission by 50% (5). In 1998, MOPH initiated a pilot program to prevent mother-infant HIV transmission in region 7, a rural area in northeastern Thailand with an antenatal HIV prevalence of approximately 1%, to assess program feasibility, effectiveness, and acceptability (Figure 1) (6). This report summarizes an evaluation of the 2-year pilot program, which indicated that acceptance of HIV testing and adherence to zidovudine were high and HIV transmission was reduced. The findings demonstrate the feasibility of implementing programs to prevent mother-infant HIV transmission on a large scale in a developing country. MOPH requested technical assistance from the HIV/AIDS Collaboration (a joint activity of MOPH and CDC) to monitor and evaluate the program. In region 7, routine antenatal counseling and voluntary confidential HIV testing were integrated into public antenatal clinic services by July 1998. HIV-infected pregnant women were offered zidovudine from 36 weeks' gestation and during labor and free powdered infant formula for 12 months. Program coverage was monitored through monthly reports collected from the antenatal and delivery departments in the 90 public hospitals in region 7, and summaries were disseminated regularly to participating hospitals, program staff, and policymakers. During July 1998--June 2000, 104,393 (86%) of 122,094 new antenatal clinic clients were tested for HIV; 964 (1%) were HIV infected (Table 1). Of 153,598 women who gave birth in the 90 region 7 hospitals during the same period, 151,928 (99%) had received antenatal care, and HIV status was documented in the delivery records of 106,834 (70%). At delivery, of 922 HIV-infected women, 640 (69%) had received antenatal zidovudine prophylaxis. Testing, documentation of HIV results at delivery, and zidovudine use increased significantly during the program period (Table 1). To evaluate the program's coverage, acceptability, and impact, two groups of women were interviewed: those who had given birth within 2 months of the interview and whose delivery record lacked documentation of HIV status and HIV-infected women who had given birth during the 12 months preceding the interview. Women were identified from hospital logs from 11 hospitals where 44% of HIV-infected women had given birth during the preceding year. All HIV-infected women and a random sample of women whose HIV status was not documented were invited by letter to attend a health-care facility. Women who agreed to participate were interviewed during April--May 2000 by trained interviewers who used structured questionnaires. Of 215 women whose HIV status was not documented at delivery, 117 (54%) reported that they had had an HIV test during pregnancy. In addition, 83 (71%) of the 117 women tested knew their HIV result, and all reported a negative test result. Of 162 HIV-infected women interviewed, 152 (94%) reported an HIV diagnosis before delivery, 159 (98%) reported that they had received posttest counseling, and 128 (79%) reported that they had taken zidovudine prophylaxis. Most women (89%) who had taken zidovudine reported not missing any doses of medication. Two (1%) women refused zidovudine prophylaxis. All HIV-infected women reported using infant formula, and 10 (6%) women reported breastfeeding for a short period. In comparison, 204 (95%) of the 215 women whose HIV status was not documented reported that they breast-fed. Of the 162 HIV-infected women, 146 (90%) reported not wanting another child, and 78 (48%) already had had a tubal ligation. Results from HIV polymerase chain reaction (PCR) tests were used to assess the program's effectiveness in preventing HIV transmission; tests were provided as a service to children born to HIV-infected women during the latter part of the program period. One or more PCR tests were performed on 293 HIV-exposed infants after age 1 month. Of these, 19 (8%) of 229 (95% confidence interval [CI]=5%--13%) infants whose mothers had received zidovudine tested HIV positive, and nine (14%) of 64 (95% CI=7%--25%) infants whose mothers had not received zidovudine tested HIV positive and were considered infected. Overall, risk for mother-infant HIV transmission was estimated at 10% (95% CI=6%--14%). Working groups periodically reviewed program data and developed strategies to strengthen program coverage, acceptability, and impact (6). On the basis of clinical trials and pilot projects in Thailand during 1996--1999, MOPH launched a national program to prevent mother-infant HIV transmission in Thailand in 2000 (5--8). Reported by: V Thaineua, S Kanshana, D Thewanda, P Amornwichet, N Kullerk, N Voramongkol, S Akksilp, V Sereesitipitak, A Pensiri, P Nimnakorn, K Chaisit, B Juengsmarn, P Phewruangnonta, S Loiha, S Piyapongkul, T Sarnthima, L Yampiwan, M Saenjai, C Paopha, Ministry of Public Health; A Teeraratkul, T Naiwatanakul, N Skunodom, K Limpakarnjanarat, The HIV/AIDS Collaboration, Nonthaburi, Thailand. Div of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention; and an EIS Officer, CDC. Editorial Note:The findings in this report indicate that interventions to reduce mother-infant HIV transmission can be implemented successfully on a large scale in Thailand. These interventions, integrated into existing maternal and child health-care services, were acceptable to most women and reduced mother-infant HIV transmission from an estimated 30% to approximately 10% (4,8). This report also highlights the rapid translation of research findings into a national public health prevention program in a developing country. Despite the implementation of antenatal HIV testing, maternal zidovudine prophylaxis, and infant formula in Thailand, these interventions have not been widely implemented in countries with high HIV prevalence. Similar programs have been initiated in several sub-Saharan countries, but acceptance of HIV testing and zidovudine prophylaxis has been low. Limited access to antenatal and HIV-related health care and limited public health infrastructure represent major challenges to large-scale efforts in many countries. The nutritional, health, and social risks associated with the early use of formula also are potential threats to maternal and child health. In settings where breast-feeding is almost universal, women who do not breast-feed may be stigmatized as HIV infected. In poor, unsanitary environments, the use of formula is associated with increased morbidity and mortality from malnutrition, diarrhea, and respiratory infections (9). In recent clinical trials, simpler, less expensive interventions using zidovudine with lamivudine or nevirapine also have prevented mother-infant HIV transmission, and these regimens might help overcome some of these barriers (10). Medications begun intrapartum, particularly nevirapine, have feasibility and cost advantages over more complex regimens and can be given to women who have received suboptimal antenatal care. CDC and other organizations are working with many developing countries to implement simple interventions to prevent mother-infant HIV transmission in other large-scale programs. Such programs will be one component of a U.S. initiative to enhance HIV prevention and care in developing countries. The pilot program in Thailand underscores the importance of monitoring and evaluating to facilitate timely program improvements and optimize the impact and acceptability of these HIV-prevention programs. The simple, focused approach to monitoring and evaluating used in Thailand provides a useful model that minimizes the workload for limited public health personnel. The findings in this report are subject to at least two limitations. First, estimates of program effectiveness are derived from the HIV test results of a nonrandom subset of infants who received tests as part of a clinical service. Second, HIV-infected women interviewed received care at large health-care facilities and responded to a general invitation letter; therefore, the results may not be generalizable to women attending smaller health-care facilities or to the 21% of HIV-infected women who did not respond to the invitation letter and attend an interview. On the basis of the estimated 20% decrease in mother-infant HIV transmission among the 15,000 infants born to HIV-infected women, the Thai national program has the potential to prevent approximately 3000 infant HIV infections each year. If similar programs were implemented worldwide, hundreds of thousands of childhood HIV infections could be prevented. In addition to reducing mother-infant HIV transmission, such programs can improve voluntary counseling and testing services, reduce the sexual transmission of HIV, promote informed decisions about childbearing, and link HIV-infected persons to health and social services. References
Table 1 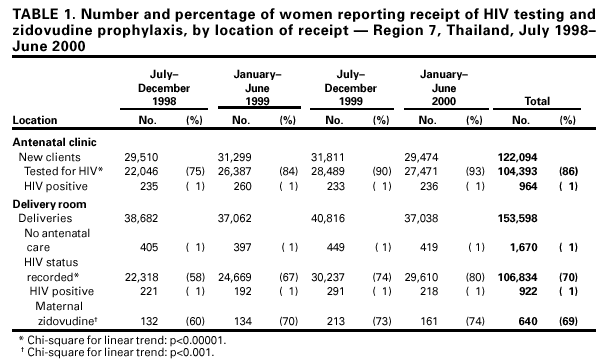 Return to top. Figure 1  Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/20/2001 |
|||||||||
This page last reviewed 7/20/2001
|