 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Public Health Dispatch: Fibrosing Skin Condition Among Patients with Renal Disease --- United States and Europe, 1997--2002During May 1997--November 2000, eight (3%) of 265 kidney transplant recipients at a hospital in California developed an unusual skin condition posttransplant (Figure 1). On clinical examination, the patients had fibrotic skin lesions histologically resembling scleromyxedema on their distal extremities and trunk, resulting in severe contractions and limited mobility. However, the usual IgG lambda paraprotein associated with scleromyxedema was not observed in these patients. Personnel in the dermatopathology section at the University of California, San Francisco, reviewed the biopsies and concluded that this skin disorder had not been described previously. As a result, health-care providers at the hospital where the index patient was treated asked the California Department of Health Services (CDHS) and CDC to assist in the investigation. This report summarizes preliminary findings from the investigation. A case was defined as large areas of hardened skin with slightly raised plaques or papules, with or without pigment alteration, in a patient with a skin biopsy indicating increased dermal fibroblasts and mucin and an abnormal dermal collagen bundle pattern. Additional patients were identified by responses to a publication describing the condition (1), by colleague referral, and by contacting members of the American Society of Dermatopathology, who were asked to alert other clinicians about the condition and to refer potential patients to CDHS. As of January 2002, 49 patients have been identified throughout the United States and Europe. Although having renal disease is not a part of the case definition, all patients have had underlying renal disease; approximately half have had renal transplantation. No consistently effective treatment exists; however, several patients have improved. To identify risk factors for this condition, in February 2001, CDHS conducted a case-control study among the eight case-patients at the index hospital, all of whom had renal disease and had undergone renal transplantation. Three controls were selected per case, matched by closest renal transplant date. Medical records for case- and control-patients were reviewed for demographic characteristics, procedures, infections, laboratory values, measures of renal function, and medication exposures. Case- and control-patients were similar demographically, in the type and duration of immunosuppressive therapy or type of pretransplant dialysis, kidney transplant type, invasive procedures (e.g., surgical or diagnostic), or posttransplant infections. Case-patients were more likely than controls to have poor renal function posttransplantation, which included requiring hemodialysis and receiving medications associated with severe disease. Because this investigation involved a small number of patients who had undergone renal transplantation, the case-control study should be expanded to include other reported cases, including cases among nontransplant patients. Clinical and histopathologic photographs of this condition are available at http://www.pathmax.com/dermweb. Information about patients with this condition can be reported to mgoveia@dhs.ca.gov until July 2002. Reported by: S Cowper, MD, Dept of Dermatology and Pathology, Yale Univ, New Haven, Connecticut. P LeBoit, MD, Dermatopathology Section, Univ of California, San Francisco. L Su, MD, Pathology Dept, Univ of Michigan, Ann Arbor. M Grossman, MD, Dept of Dermatology, Columbia Presbyterian Medical Center, New York, New York. G Windham, PhD, D Gilliss, MD, E Wersinger, MPH, Environmental Health Investigations Br, California Dept of Health Svcs. W Jarvis, MD, Div of Healthcare Quality Promotion, National Center for Infectious Diseases; and M Goveia, MD, EIS Officer, CDC. Reference
Figure 1 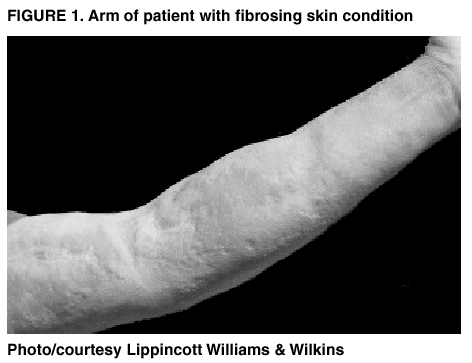 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/17/2002 |
|||||||||
This page last reviewed 1/17/2002
|