 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Measles Epidemic Attributed to Inadequate Vaccination Coverage --- Campania, Italy, 2002In Italy, measles has been a mandatory reportable disease for >100 years. During the prevaccination era, approximately 25,000--90,000 cases were reported annually. During the late 1980s and 1990s, incidence declined with increasing measles vaccination coverage, but measles epidemics continued to occur periodically, most recently during 1995--1997 (Figure 1). In early 2002, measles incidence increased sharply; the area most affected was Campania (2001 population: approximately 5,782,000, including 1,100,000 children aged <15 years), a large region in southern Italy (Figure 2). In 2001, estimated measles vaccination coverage for the 1998 Campania birth cohort was 65% (1). Regional health authorities and the National Institute of Health investigated the measles outbreak in Campania. This report summarizes the preliminary results of the investigation, which attributed the epidemic to inadequate vaccination coverage. A coordinated effort is needed to interrupt measles transmission in Italy. To monitor the incidence of measles and other vaccine-preventable diseases among children aged <15 years, in January 2000, a national pediatric sentinel surveillance system (SPES) was initiated, covering approximately 4% of children aged <15 years (2). Children are tracked by National Health System primary-care pediatricians who participate voluntarily. These pediatricians report cases of measles and other vaccine-preventable diseases diagnosed during the preceding month by e-mail or fax to the National Institute of Health in Rome. SPES uses a standard clinical case definition for measles (3), but laboratory confirmation is not required. Information collected on each case includes date of birth, date of illness onset, sex, and vaccination status. Reporting on presence or absence of cases is required, and the monthly incidence rate is calculated by using the number of reported cases as the numerator and the total patient population cared for by the pediatricians participating in the surveillance system during that month as the denominator. Data on measles-associated hospitalizations that occurred during January--July 2002 were collected by reviewing discharge records of the five main regional hospitals with infectious diseases units. For each patient, information about sex, date of birth, admission and discharge dates, and diagnosis at discharge was collected. Information on regional vaccination coverage was derived from a survey conducted during 2000--2001 of a sample of 12,647 children aged 24--36 months who were born in 1998 (1). In 2002, an average of 51 pediatricians in Campania participated in SPES, covering an estimated 41,888 children (3.8% of the regional total). A total of 1,571 measles cases were reported, resulting in an annual incidence of 3,750 cases per 100,000 children aged <15 years. The majority (1,543 [98%]) of cases occurred during January--July, with a peak in May (Figure 3). Incidence increased with age, from 1,088 per 100,000 in children aged <1 year to 2,413 in those aged 1--4 years, 3,506 in those aged 5--9 years, and 5,592 in those aged 10--14 years. Incidences were highest in the provinces of Naples and Caserta, the areas with the lowest measles vaccination coverage (Table). Vaccination status was known for 1,543 (98%) of the reported patients; of these, 101 (7%) were vaccinated, and the remainder were unvaccinated. The number of administered doses was reported for 72 (71%) children; 70 were vaccinated with 1 dose, and two with 2 doses. The proportion of vaccinated patients aged >1 year decreased with age, from 10.5% among children aged 1--4 years to 5.5% among children aged 5--14 years. Hospital record review identified 594 measles-associated hospitalizations that occurred during January--July 2002. Of these, 469 (79%) occurred among children aged <15 years, and 44 (7%) in infants aged <1 year. Diagnosis at discharge was available for 425 (91%) children; 99 (23%) had respiratory complications (pneumonia/bronchopneumonia), 12 (3%) encephalitis, and two (1%) thrombocytopenia. Of the remaining children, 15 (4%) had other complications, and 297 (70%) had uncomplicated measles. Three children (aged 6 months, 4 years, and 10 years, respectively) died. The remaining 125 (21%) cases of identified measles-associated hospitalizations occurred among persons aged >15 years, including three additional cases of encephalitis and one measles-associated death attributed to respiratory failure in a person aged 29 years. Regional health authorities recommended 1) vaccinating persons exposed to patients in family and school settings; 2) offering measles, mumps, and rubella (MMR) vaccine to all persons who had not been vaccinated or who did not have a history of measles; and 3) temporarily lowering the age of MMR vaccination to 6 months, with subsequent revaccination after 1 year of those children vaccinated at age 6--12 months. However, these measures did not stop the epidemic, which spread subsequently to other areas. Approximately 1,000 additional cases were reported to SPES from other regions, and the national annual attack rate in children aged <15 years was 738 per 100,000. The most affected regions were central and southern Italy, with regional incidences seven to 36 times higher than in northern Italy. After an apparent decline in reported incidence during August--December 2002, the epidemic continued during the first half of 2003, affecting the southern regions of Abruzzo, Puglia, and Calabria (Figure 2) (4). Reported by: ML Ciofi degli Atti, MD, F Fabi, MS, S Salmaso, D Biol, National Center for Epidemiology, Surveillance, and Health Promotion, National Institute of Health. R Pizzuti, MD, E de Campora, MD, Regional Health Authority, Campania, Italy. Editorial Note:Four measles-associated deaths and 594 hospitalizations occurred during January--July 2002 in Campania. This outbreak indicates that measles can be severe and sometimes fatal, even in industrialized countries. The outbreak occurred as a result of low vaccination coverage and affected primarily unvaccinated school-aged children. Vaccination coverage levels were lower for school-aged children than for preschool-aged children. The regional measles vaccination coverage estimated for the 1991 birth cohort was 16% in 1993 (5), increasing to 65% for the 1998 birth cohort (1). Inadequate vaccination coverage could not interrupt virus circulation but resulted in a prolonged interepidemic interval (an earlier epidemic in Campania occurred in 1996) and a shift of the disease incidence toward older age groups during the 2002 epidemic. The findings in this report are subject to at least two limitations. First, although SPES is four times more sensitive than statutory notification in detecting measles cases at the national level, and 22 times more sensitive in southern Italy (1), it obtains data only on children aged <15 years. Because incidence data on older adolescents and adults were lacking, the extent of the epidemic probably was underestimated, and biased age distribution of cases probably occurred. Because incidence increased with age and peaked among children aged 10--14 years, many cases probably occurred among persons aged >15 years, which is consistent with data obtained through the hospital record review. Second, provincial results should be interpreted cautiously. SPES was designed to obtain information at the regional level. Although the large number of children in Campania under surveillance permitted estimation of incidence figures for each province, not all provinces were represented equally. In Italy in 1979, measles vaccination was recommended for children aged >15 months. During the early 1990s, combined MMR vaccines were introduced, and in 1999, the recommended age of administration was lowered to 12--15 months. In areas where vaccine coverage among infants aged <2 years was >80%, administration of a second dose at age 5--6 years or at age 11--12 years has been recommended since 1999. However, each of the country's 20 regions establishes its own measles vaccination policy, and adherence to recommendations has not been universal. As a result, national vaccination coverage with 1 dose of MMR vaccine by age 24 months remains inadequate, with an estimated coverage of 74% in 2001 (6). Coverage is lowest in southern Italy (7). Italy's Field Epidemiology Training Program (FETP, known locally as PROFEA) assisted in this investigation. Modeled after CDC's Epidemic Intelligence Service, PROFEA was created to establish an experienced group of epidemiologists at local and regional levels. Measles elimination requires achieving and sustaining 2-dose coverage of >95% in multiple subsequent cohorts, either through routine vaccination (8) or a combination of routine and supplemental immunization activities (9). The World Health Organization's European Region aims to eliminate measles by 2007, but large differences in vaccination coverage and disease incidence exist among European countries (10). In Italy, the interruption of measles transmission can be achieved at the national level only with coordinated and uniform actions throughout the country. For this reason, a national plan has been developed jointly by regional health authorities, the National Institute of Health, and the Ministry of Health. Key strategies to achieve measles elimination in Italy include 1) improving routine coverage with 1 dose of MMR vaccine to >95% of children aged 24 months, 2) conducting a national "catch-up" vaccination campaign for children aged 6--13 years during 2004--2005, 3) achieving and sustaining a high coverage with a second routine dose of MMR vaccine among children aged 5--6 years while administering the MMR vaccine simultaneously with the DTaP booster dose included in the national schedule, and 4) strengthening surveillance. References
Figure 1 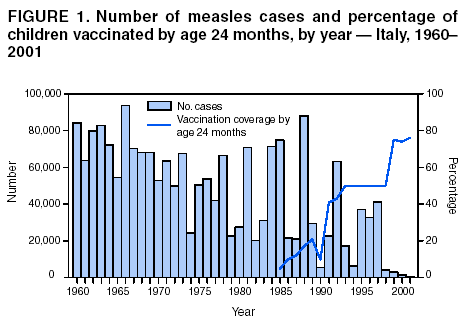 Return to top. Figure 2 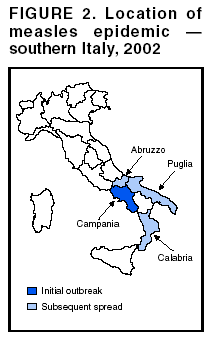 Return to top. Figure 3 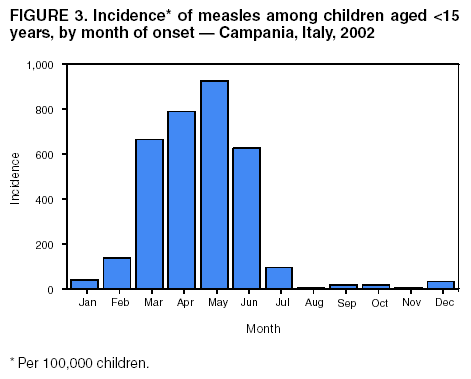 Return to top. Table 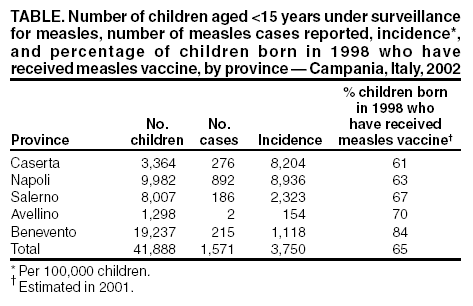 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/30/2003 |
|||||||||
This page last reviewed 10/30/2003
|