 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Investigation of a Ricin-Containing Envelope at a Postal Facility --- South Carolina, 2003On October 15, 2003, an envelope with a threatening note and a sealed container was processed at a mail processing and distribution facility in Greenville, South Carolina. The note threatened to poison water supplies if demands were not met. The envelope was isolated from workers and other mail and removed from the facility, and an investigation was begun. On October 21, laboratory testing at CDC confirmed that ricin was present in the container. To assess the human health effects related to possible ricin exposure, the South Carolina Department of Health and Environmental Control (SCDHEC) and CDC interviewed all workers at the postal facility and initiated statewide surveillance for illness consistent with ricin exposure during October 15--29. On October 22, the facility was closed for a detailed epidemiologic and environmental investigation. This report summarizes the results of the investigation, which found no evidence of environmental contamination and no cases of ricin-associated illness. Clinicians and public health officials should be vigilant for illnesses suggestive of ricin exposure. SCDHEC asked emergency departments, clinicians, health departments, and the local postal facility to report any cases consistent with ricin exposure to the state health department and CDC. State poison control center records and intensive care unit charts at seven hospitals in the Greenville, Spartanburg, and Anderson areas were reviewed daily for illness consistent with ricin exposure. A CDC medical toxicologist and state and local health department epidemiologists interviewed all 36 workers at the postal facility to identify ricin-related illnesses. CDC conducted environmental assessment and sampling at the postal facility, consisting of 70 wipe samples and five surface dust samples (collected by sampling pumps and sampling filter media). Wipe samples were obtained by using Dacron swabs moistened with sterile buffered solution and were collected from specific surfaces in the facility, including storage bins, surfaces, conveyor belts, and sorting tables that had been in contact with the letter. All environmental samples were analyzed at CDC and were negative for ricin. No workers had illness suggestive of ricin exposure. Statewide surveillance did not identify any cases of ricin-associated illness. However, two cases of multisystem organ failure and several nonspecific illnesses, which likely were detected because of increased surveillance and reporting, were investigated within the state. The postal facility was reopened after 1) all workers who had worked at the facility since the package was discovered had been contacted and confirmed to be well and 2) environmental samples for ricin were negative. As of November 19, no ricin-associated cases had been identified. Regional and national surveillance for illness consistent with ricin poisoning was initiated through an ongoing collaboration between CDC, ATSDR, and the American Association of Poison Control Centers' Toxic Exposure Surveillance System (TESS). Surveillance for potential cases was accomplished by monitoring call volumes at 62 of the 63 poison control centers in the United States for clinical effects consistent with ricin poisoning and for cases referring to the specific product code ("Contaminated Water") because water had been stated as a potential target by the note in the package. During October 15--29, approximately 97,000 human exposure calls were reported to TESS. No ricin-associated syndromes or events were identified. Reported by: J Gibson, MD, D Drociuk, MSPH, T Fabian, MD, S Brundage, MD, L Ard, N Fitzpatrick, MPH, W Moorhead, JD, South Carolina Dept of Health and Environmental Control. M Schwartz, MD, E Kilbourne, MD, ATSDR; J Schier, MD, M Patel, MD, M Belson, MD, C Rubin, DVM, Div of Environmental Hazards and Health Effects, J Osterloh, MD, Div of Laboratory Sciences, S Deitchman, MD, National Center for Environmental Health; Max Kiefer, CIH, National Institute for Occupational Safety and Health, R Meyer, PhD, Bioterrorism Preparedness Response Program, National Center for Infectious Diseases, CDC. Editorial Note:The Federal Bureau of Investigation (FBI) and local law enforcement authorities are conducting an investigation to identify the illegal source of this toxin. However, until a source is identified and eliminated, health-care providers and public health officials must consider ricin to be a potential public health threat and be vigilant about recognizing illness consistent with ricin exposure. Ricin is a biologic toxin derived from the castor bean plant Ricinus communis (1,2) (Box.) Ricin is one of several toxalbumins that exert toxicity by inhibiting protein synthesis in eukaryotic cells (1,2). Several instances of ricin procurement for use as a terrorist weapon have been documented (3--5). Routes of exposure to ricin include ingestion, inhalation, parenteral, dermal, or ocular; however, systemic toxicity has been described in humans only after ingestion or injection. Ricin is considered to be a much more potent toxin when inhaled or injected compared with other routes of exposure. Ricin poisoning is not contagious, and person-to-person transmission does not occur. Processed and purified ricin can be disseminated by aerosol, contamination of food or water, or injection (1,6). Ricin particles of <5 microns have been used for aerosol dispersion in animal studies and can stay suspended in undisturbed air for several hours. Resuspension of settled ricin from disturbed surfaces also might occur. Data about the effects of ricin poisoning on humans are limited. Because ricin poisoning might resemble typical gastroenteritis or respiratory illness, it might at first be difficult to discern from other illnesses. For this reason, suspicion of cases should occur in conjunction with epidemiologic clues suggestive of chemical release (e.g., an unusual increase in the number of patients seeking care or unexpected progression of symptoms in a group of patients) or a credible threat of chemical release in the community (7). As in the instance described in this report, health departments should inform clinicians, poison control centers, and other health departments rapidly of any emerging evidence of ricin exposures. Clinical ManifestationsIngestion: No reports of illness after ingestion of purified ricin toxin have occurred. Signs and symptoms from oral exposure to purified ricin are presumed to be similar to reports of illness after castor bean mastication and ingestion (6). However, reports of illness from castor bean ingestion also are not well documented. Toxicity can range from mild to severe and can progress to death (6). Mild illness can include nausea, vomiting, diarrhea, and/or abdominal cramping. Onset of gastrointestinal symptoms typically occurs in 1--4 hours (6). In moderate to severe illness, gastrointestinal symptoms (i.e., persistent vomiting and voluminous diarrhea [bloody or non-bloody]) typically lead to substantial fluid loss, resulting in dehydration and possibly hypovolemic shock (6). In severe poisoning, liver and renal failure and death are possible. Inhalational Exposure: Data on inhalational exposure to ricin in humans are limited. Workers exposed to castor bean dust have described allergic reactions (e.g., nasal and throat congestion, eye irritation, hives, chest tightness, and wheezing) (8). Aerosol exposures to ricin can be followed within 4--8 hours by fever, chest tightness, cough, dyspnea, nausea, and arthralgias followed by diaphoresis (9). Parenteral Exposure: In a single human trial evaluating low doses of intravenous ricin as a chemotherapeutic agent, influenza-like symptoms with fatigue and myalgias for several days were reported (1). Ricin injection in one case caused weakness within 5 hours, fever and vomiting within 24 hours, followed by shock and multi-organ failure, and death in 3 days (1). Management and DecontaminationTreatment for ricin toxicity is primarily supportive, including intravenous fluids, vasopressors, respiratory support, and cardiac monitoring. No specific antidotal therapy exists, and ricin cannot be removed by dialysis. Prophylactic vaccine and immunotherapy are not available (1). The same general guidelines for gastrointestinal decontamination employed for other ingested toxins should be applied to ricin (10). A single dose of activated charcoal should be administered as soon as possible if the patient is suspected of ricin ingestion and is not vomiting. The efficacy of gastric lavage is controversial but may be considered for known or suspected substantial ingestions if presentation to the hospital occurs within 1 hour of ingestion. Ipecac, whole bowel irrigation, and cathartics should not be used routinely for known or suspected ricin poisoning. Clinical presentations and their management can vary considerably. Clinicians are strongly advised to contact their regional poison control center immediately upon suspicion of a case of ricin exposure for guidance and further individualized management. Skin decontamination for ricin exposure should be performed if a powder or similar substance is found on the patient, preferably in a designated area outside the main emergency department. Potentially exposed persons should be advised to wash their hands thoroughly with soap and water and refrain from any hand-to-mouth activities. Laboratory DetectionNo methods are available for the detection of ricin in biologic fluids. Ricinine is a separate compound from ricin present in the castor bean and might be more feasible to monitor in persons exposed to ricin-containing plant material. Preparations of ricin-containing substances and environmentally collected specimens can be tested for the presence of ricin by a time-resolved fluorescence immunoassay, available at CDC and member Laboratory Response Network state public health laboratories. In addition, CDC performs a polymerase chain reaction assay on similar type specimens that will detect the gene in the plant material that codes for the ricin protein. Several commercial handheld or test-strip detection devices are available, but the performance of these assays is unknown. ReportingSuspected or known cases of ricin poisoning should be reported immediately to the regional poison control center (telephone, 1-800-222-1222) and to local or state public health agencies, which will report cases to other health departments, CDC, and other federal agencies. References
Box 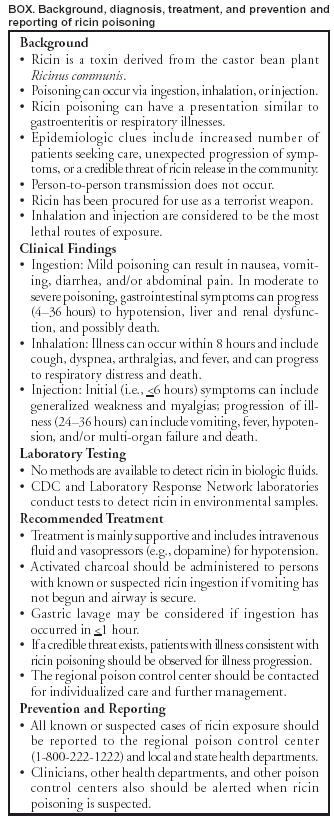 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/20/2003 |
|||||||||
This page last reviewed 11/20/2003
|