 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Diminishing Racial Disparities in Early-Onset Neonatal Group B Streptococcal Disease --- United States, 2000--2003Increased use of intrapartum antibiotics to prevent perinatal group B streptococcal (GBS) disease during the 1990s led to substantial declines in the incidence of GBS disease in newborns (1). Despite this success, at the end of the 1990s, early-onset GBS disease (in infants aged <7 days) continued to be a leading infectious cause of neonatal mortality in the United States, and black infants remained at higher risk than white infants (1). In 2002, CDC and the American College of Obstetricians and Gynecologists (ACOG) revised guidelines for prevention of early-onset GBS disease to recommend late prenatal screening of all pregnant women and intrapartum antibiotic prophylaxis (IAP) for GBS carriers (2,3). These guidelines were expected to result in further declines in early-onset disease (4). This report updates early-onset incidence trends since 1999 analyzed by using population-based, multistate data from the Active Bacterial Core surveillance (ABCs)/Emerging Infections Program Network. The results of the analysis indicated that 1) after a plateau in early-onset disease incidence during 1999--2002, rates declined 34% in 2003 and 2) although racial disparities in incidence persist, rates for blacks now approach the 2010 national health objective of 0.5 cases per 1,000 live births (5). Continued implementation of screening and prophylaxis guidelines by clinicians and public health practitioners should lead to further declines in racial disparities. ABCs conducts active, laboratory-based surveillance for all cases of invasive GBS, including periodic audits to ensure completeness of case finding. A case of early-onset GBS disease was defined as isolation of GBS from a normally sterile site (e.g., blood or cerebrospinal fluid) in a neonate aged 0--6 days residing in an ABCs area. Participating areas during 2000--2003 were Connecticut, Maryland, Minnesota, and selected counties in California, Colorado (beginning in 2001), Georgia, New York, Oregon, and Tennessee, representing a population that produced 419,062 live births in 2001. Of the 2001 live-birth cohort, 73% were white, 20% were black, and 7% were of other races; 15% were of Hispanic origin. The incidence of early-onset disease was calculated by using live-birth data for 2000 and 2001 from ABCs states' vital statistics or the National Vital Statistics Report (available at http://www.cdc.gov/nchs/data/nvsr/nvsr51/nvsr51_02.pdf). Incidence for 2002 and 2003 were calculated by using 2001 live-birth data. Incidence of GBS disease from earlier surveillance years was derived from data published previously (1) using comparable methods. A total of 184 (13.2%) of 1,397 cases with missing or unspecified race data during 1996--2002 were matched with birth records to improve the completeness of race reporting. Remaining cases of unknown race (during 1996--2002, a total of 77 [5.5.%] of 1,397; in 2003, a total of 21 [15.7%] of 134) were distributed on the basis of the known race distribution within each county and included in all reported rates. To assess the impact of the August 2002 guidelines, incidence in 2003 was compared with the average incidence for 2000 and 2001; 2002 was considered a transition year. During 2000--2003, a total of 701 cases of early-onset GBS disease were reported in the surveillance areas (Table). Outcome was known for 676 (96.4%) cases; the case-fatality ratio was 6.5%. A total of 150 (21.4%) infants were born before 37 weeks' gestation; among these preterm infants, the case-fatality ratio was 22.7%. During 1999--2001, early-onset disease incidence remained nearly constant, with an average of 0.47 cases per 1,000 live births. In 2003, the overall disease incidence was 0.32 (Figure 1), representing a 34% (95% confidence interval [CI] = 20%--46%) decline in incidence since 2000--2001. The incidence in 2003 varied geographically, from 0.53 in Tennessee to 0.14 in Oregon (Table). Rates in Georgia decreased significantly compared with the 2000--2001 baseline (p<0.01), and rates in Tennessee decreased marginally (p = 0.06). During 1999--2001, disease incidence remained stable for both black and white populations, and rates among black infants were approximately twice those of whites (Figure 2). In 2003, the incidence of disease was 0.26 cases per 1,000 live births among white infants, 0.59 among black infants, and 0.16 among infants of other races; the rate among those of Hispanic origin was 0.31. Compared with disease rates in 2000 and 2001, the incidence of disease in 2003 declined 34% among white infants and 30% among black infants. However, black infants remained 2.2 (95% CI = 1.6--3.2) times more likely to have early-onset GBS disease than white infants in 2003; this relative risk has not changed significantly since 1996 (Figure 2). Compared with the pre-prevention baseline rate in 1993, the difference in incidence between whites and blacks has declined 68% (i.e., by 0.78 cases per 1,000 births). In 1998, white neonates achieved the 2010 national health objective (5); preliminary data from 2003 indicate that black neonates are approaching this goal. Reported by: S Brooks, MPH, M Apostol, MPH, J Nadle, MPH, A Grey, MPH, California Emerging Infections Program, Oakland, California. N Haubert, S Burnite, T Crume, MSPH, Emerging Infections Program, Colorado Dept of Public Health. NL Barrett, MS, Emerging Infections Program, Connecticut Dept of Public Health. MM Farley, MD, P Martell-Cleary, MSW, Georgia Emerging Infections Program, Veterans Affairs Medical Center and Emory Univ School of Medicine, Atlanta, Georgia. L Harrison, MD, LT Sanza, Maryland Emerging Infections Program, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland. C Morin, MPH, R Lynfield, MD, Minnesota Dept of Health. G Smith, Emerging Infections Program, New York State Dept of Health. P Cieslak, MD, K Stefonek, MPH, Oregon Dept of Human Svcs. B Barnes, Vanderbilt Univ School of Medicine, Nashville; AS Craig, MD, Tennessee Dept of Health. SI McCoy, MPH, S Schrag, DPhil, A Schuchat, MD, Div of Bacterial and Mycotic Diseases; KA Robinson, MPH, Office of Surveillance, Active Bacterial Core surveillance/Emerging Infections Program Network, National Center for Infectious Diseases, CDC. Editorial Note:Although the incidence of early-onset GBS disease declined during the 1990s (1,2,6), disease incidence plateaued until 2002, when universal screening guidelines were issued. The rate in 2003 of 0.32 cases per 1,000 live births is the lowest ever recorded for the United States and meets the 2010 national health objective for overall incidence (5), with all of the ABCs areas at or below the target of 0.5 cases per 1,000 live births. A 2002 review of a random sample of live births in ABCs areas estimated that, under universal screening, the overall incidence of early-onset infections would be approximately 0.3 cases per 1,000 live births (4); the data from 2003 are consistent with this prediction. These data likely underestimate the full impact of guidelines released in the latter half of 2002, because institutions following the old risk-based guidelines were unlikely to have completed the transition to universal screening. In addition, improved implementation of screening through optimal prenatal specimen collection and processing; improved communication between laboratories and providers; and appropriate choice of prophylactic agents, particularly for penicillin-allergic women, might lead to further declines in disease incidence. Moreover, clinical laboratories have improved in GBS isolation and processing since the 1996 guidelines; however, opportunities to improve the implementation of recommendations related to antimicrobial susceptibility testing and GBS bacteriuria were identified (7). Although record low rates of early-onset GBS disease were recorded for black neonates in 2003, racial disparities persist. The reasons for higher rates of neonatal GBS disease among blacks are multifactorial. A key factor is substantially higher GBS colonization rates among blacks; in addition, preterm delivery is more common in blacks and increases risk for both early- and late-onset GBS disease (8). Increased GBS prevention efforts during the 1990s coincided with a 75% reduction in the difference in disease incidence between black and white infants (1). However, starting in 1999, racial disparities in early-onset disease plateaued. Declines in the rate of disease in black infants after release of the 2002 guidelines and new progress towards the 2010 national health objective might indicate that a universal screening strategy will further reduce this racial disparity. The findings in this report are subject to at least three limitations. First, no data on the strategy providers followed are available, so trends cannot be directly attributed to particular prevention practices. Second, race data were collected from the medical record rather than self-reports. The completeness of race ascertainment was improved through the use of birth certificate data; however, 9% of cases had unknown race reported. Finally, live-birth data were not yet available for 2002 and 2003, so exact denominators for incidence calculations could not be used. To maximize prevention, correct implementation of the screening approach is crucial. Practical tools to assist with monitoring prevention implementation have been published (9,10); additional health communication tools have been created to assist both clinicians and public health practitioners with GBS education and policy issues. These resources include a recently designed website (http://www.cdc.gov/groupbstrep) with entry portals for clinicians, clinical microbiologists, the general public, and state health departments. In addition, a new consumer education brochure designed to reach black women is available free of charge by writing CDC's Respiratory Diseases Branch at 1600 Clifton Road, N.E., mailstop C-23, Atlanta, GA 30333, by faxing requests to 404-639-3970, or by ordering online at http://www.cdc.gov/groupbstrep. Acknowledgments This report is based in part on contributions by P Daily, MPH, California Emerging Infections Program, Oakland; J Mohle-Boetani, MD, California Dept of Health Svcs. K Gershman, MD, Colorado Dept of Public Health. JL Hadler, MD, S Petit, MPH, MZ Fraser, Emerging Infections Program, Connecticut Dept of Public Health. W Baughman, MSPH, Emerging Infections Program, Veterans Affairs Medical Center, Atlanta, Georgia. Maryland Active Bacterial Core surveillance, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland. L Triden, B Jewell, J Rainbow, MPH, R Danila, PhD, Minnesota Dept of Health. N Spina, MPH, B Anderson, PhD, Emerging Infections Program, New York State Dept of Health. M Dragoon, A Zeigler, Multnomah County Health Dept, Portland, Oregon. W Schaffner, MD, Vanderbilt Univ School of Medicine, Nashville, Tennessee. R Facklam, PhD, TH Skoff, MS, C Whitney, MD, C Wright, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, CDC. References
Figure 1 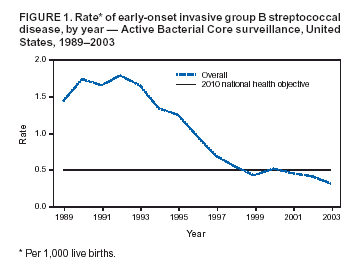 Return to top. Figure 2 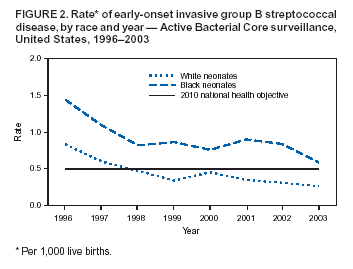 Return to top. Table 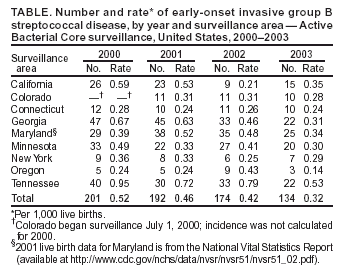 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/17/2004 |
|||||||||
This page last reviewed 6/17/2004
|